![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
3 Structure of Nucleotides
3.1 Introduction
As can be inferred from hydrolysis of nucleic acids, a monomer unit of these biopolymers includes a phosphoric acid residue in addition to that of a nucleoside. Heterocyclic base, pentose and phosphoric acid residues are present in any nucleic acid in strictly equimolar amounts; hence, in a monomer, too, there is a mole of phosphoric acid for every mole of the nucleoside. Since acid hydrolysis of monomers under certain conditions gives pentose phosphates and a heterocyclic base, the linkage between the phosphate group and nucleoside is evidently via pentose. The ester type of bonding between the phosphate group and pentose seems to be the most probable of all possible ones, with the phosphate group being linked to one of the hydroxyls, such as 5'-hydroxyl.
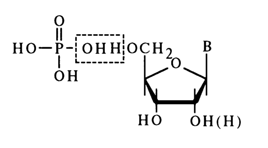
The phosphomonoester type of bonding in nucleic acid monomers is corroborated both by their chemical properties and by their behavior in the presence of enzymes specifically cleaving phosphomonoester bonds. Enzymes are known, namely, phosphomonoesterases (PME), that break down phosphonucleosides to phosphoric acid and nucleosides. It has already been mentioned that acid hydrolysis of a phosphonucleoside gives pentose phosphate, but if hydrolysis is conducted under mild conditions at pH 4 (phosphomonoester bonds at this pH value are most labile as a rule), dissociation to a nucleoside and phosphoric acid takes place. Shown below are the corresponding transformations involving adenosine phosphate.
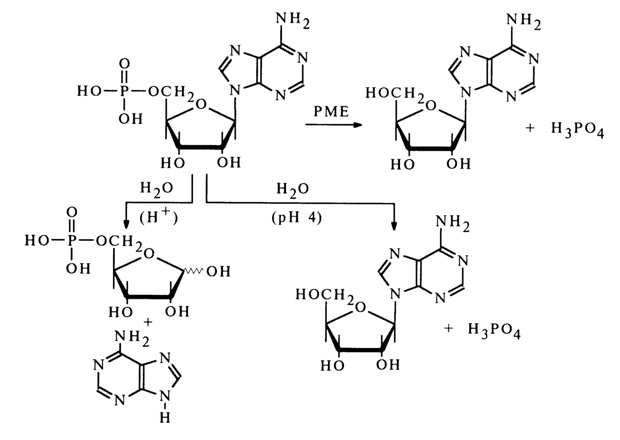
Phosphates of nucleosides are referred to as nucleotides. Depending on the structure of the pentose involved, distinction is made between ribonucleotides (monomer units of RNA) and deoxyribonucleotides (monomer units of DNA). According to the structure of the heterocyclic base constituting the nucleotide, there are purine and pyrimidine nucleotides.
Nucleotides, just as nucleosides, are usually isolated from nucleic acids after chemical or enzymatic hydrolysis. Ribonucleic acids break down to a mixture of ribonucleoside 3'- and 2'-phosphates after the treatment with a 0.3 N potassium hydroxide solution (37'C, 18 hours). Boiling with 0.1 N HCI gives pyrimidine nucleoside 3'(2)-phosphates (hydrolysis of purine nucleotides under these conditions proceeds at the glycosidic bond). Ribonucleoside 5'-phosphates result from treatment of RNA with the enzyme phosphodiesterase (PDE) obtained from snake venom, yeasts, and other sources. Deoxyribonucleotides are obtained only through enzymatic hydrolysis (digestion) of DNA, because chemical hydrolysis proceeds with complications; usually, PDE from snake venom is needed for breaking down DNA to deoxynucleoside 5'-phosphates. Under the action of PDE isolated from spleen, DNA as well as RNA yields the corresponding nucleoside 3'-phosphates. Separation of nucleotides is possible by ion-exchange and thin-layer chromatography, paper chromatography, and electrophoresis. Nucleotides are identified with the aid of UV spectra and also qualitative reactions with the phosphate group.