![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
2.5 Properties of Pseudouridine
The glycosidic C-C bond linking the ribose and uracil in pseudouridine exhibits properties differing widely from those of the ordinary N-glycosidic bond. It is highly stable in an acid medium, which is why, as has already been mentioned, serious difficulties were encountered in establishing the structure of pseudouridine by usual methods: it took a long time before the structure of the carbohydrate and pyrimidine moieties were elucidated, along with the linkage between the two. Treatment with an acid under vigorous conditions leads to isomerization rarely observed in N-glycosides. The unusual properties of the glycosidic bond stem from the allyl nature of the glycosidic carbon, which facilitates heterolytic rupture of the oxygen-glycosidic carbon bond.
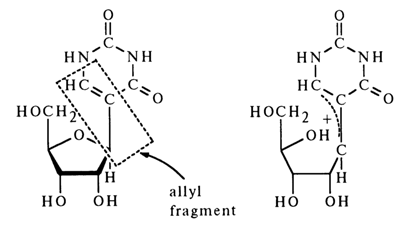
Among the factors contributing to the delocalization of the positive charge in ion (15) is also the N1 atom, which may be represented using the following structures:
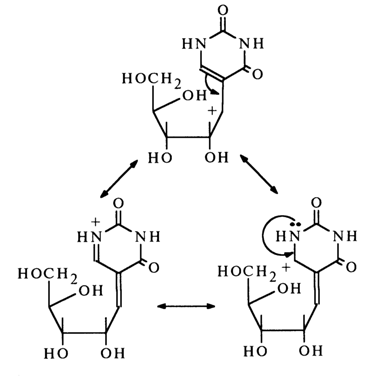
It is the relative ease with which the glycosidic carbon-oxygen bond in pseudouridine undergoes a heterolytic rupture with subsequent transformations of the emerging ion (15) that accounts for the tendency for isomerization, displayed by this nucleoside, and the structure of the resulting compounds.
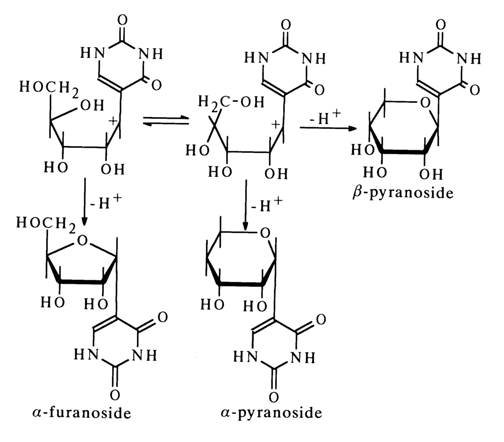
The formation of a-furanoside is understandable if it is taken into account that the carbonium center (at the glycosidic carbon) in ion (15) is planar and may be subjected to nucleophilic attack by the oxygen of the secondary hydroxyl group on either side of the plane. The result is, in one case, the starting pseudouridine having a b-configuration and, in another, its anomer
Similar factors are also involved in the formation of anomeric pyranosides, except that in this instance it is the oxygen of the primary hydroxyl group that acts as the nucleophilic agent.
The above isomerization mechanism is borne out by the fact that a similar process is observed in cases where the 2'- or 3'-hydroxyl groups are protected.
At the same time, pseudouridine derivatives at the 5'-hydroxyl group are isomerized in an acid medium to yield only a-furanosides (without formation of a- and b-pyranosides):
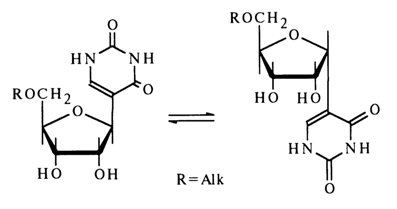
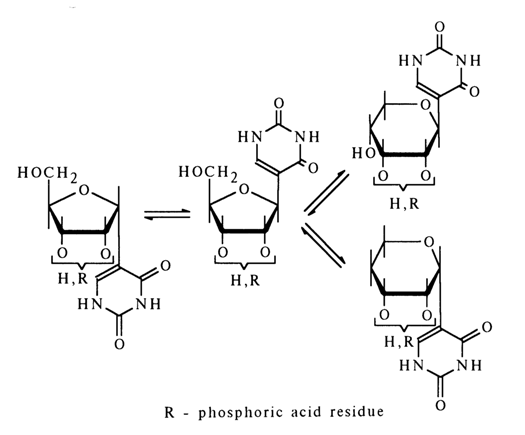
Pseudouridine also undergoes isomerization in alkaline media with formation of all possible isomers. The mechanism of this process can be written as follows:
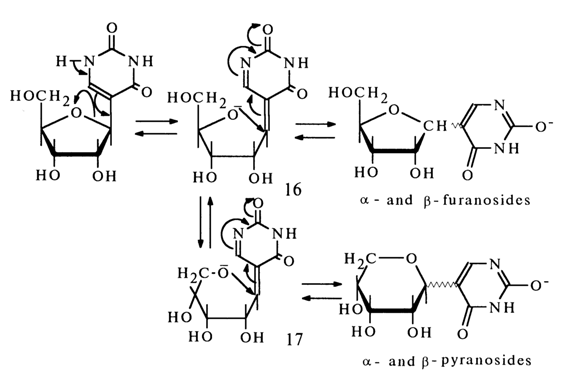
The reaction proceeds as addition of alcohols to a,b-unsaturated carbonyl compounds in the presence of bases, for example:
Addition at the multiple C-C bond may involve both primary and secondary alcohol groups [in forms (16) and (17), respectively]. Since the glycoside center in forms (16) and (17) has a two-dimensional configuration (being of ethylene type), addition of the primary hydroxyl gives a-and b-pyranosides, whereas that of the secondary hydroxyl results in a- and b-furanosides.
Just as pyrimidine nucleosides, pseudouridine is hydrogenated over a rhodium catalyst. However, in contrast to N-glycosides typically subject to addition of hydrogen at the double C5-C6 bond, it is the ether bond that undergoes hydrogenolysis in the case of pseudouridine, the double C5-C6 bond not being affected at all:
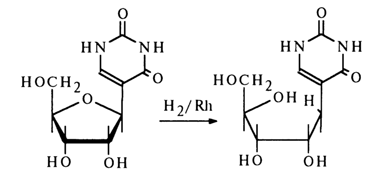
70 2 Properties of Nucleosides
This process is similar to hydrogenolysis of allyl and benzyl ethers:
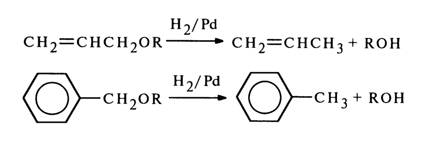
Consequently, even in this case the behavior of pseudouridine closely resembles that of compounds of the allyl type.