![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
2.4 Stability of N-Glycosidic Bonds
The N-glycosidic bonds in major nucleosides isolated from RNA and DNA are usually highly stable in neutral and alkaline media and prone to hydrolysis in the presence of mineral and organic (HCOOH, CH3COOH, CCl3COOH) acids. The hydrolysis rate depends on the hydrogen ion concentration; the reaction proceeds according to the general scheme:
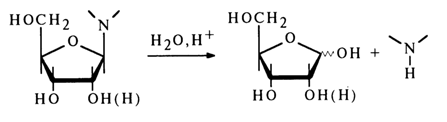
The ease of hydrolysis is largely dependent on the nature of the heterocyclic base and sugar moiety.
2.4.1 Effect of the Heterocyclic Base SpeciesAcid hydrolysis of purine derivatives is much faster than of pyrimidine ones, which becomes evident from the following kinetic data on nucleoside hydrolysis (pH 1.0, 370C):
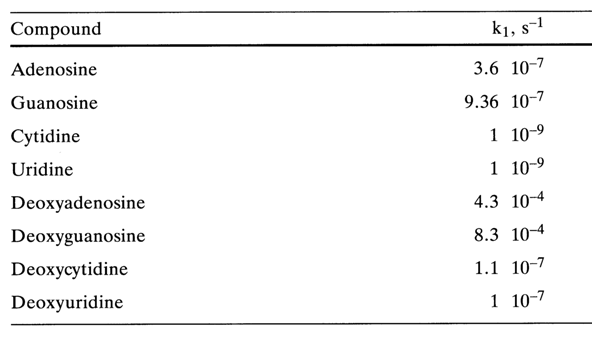
For hydrolysis of purine ribonucleosides, heating with 0.1 N hydrochloric acid (1000 C, 1 hour) is quite sufficient, in contrast, pyrimidine nucleosides requires boiling with 3 N HCI (1250 C, 4 hours).
The hydrolysis rate is also markedly affected by substituents in the heterocyclic ring.
Glycosidic bonds in guanine derivatives are more sensitive to acids, as compared to those in adenine derivatives. Substitution of hydroxy groups for the amino ones in these nucleosides leads to a perceptible labilization of the glycosidic bonds. For instance, derivatives of such a hydroxy analogue of guanine as xanthine are hydrolysed more easily than those of hypoxanthine which is a hydroxy analogue of adenine.
Introduction of alkyl groups at positions 3 and 7 of the purine ring (alkylation of nitrogens N3 and N7) also causes a tangible decrease in the glycosidic bond strength. A case in point is 7-methyldeoxyguanosine
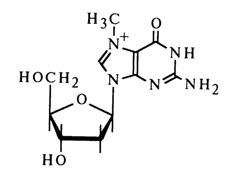
whose hydrolysis under mild conditions proceeds at a rate ten thousand times faster than in the case of deoxyguanosine. N3- and NI-methyl derivatives of deoxyadenosine are much less stable than the latter.
In the case of pyrimidine nucleosides, as opposed to purine ones, substitution of a hydroxy group for the amino group at position 4 renders the glycosidic bond much more stable. As can be inferred from the date that follow, under conditions where deoxyuridine remains virtually unchanged most of deoxycytidine is hydrolysed (hydrolysis with 5 % trichloroacetic acid, 1000 C, 30 min):
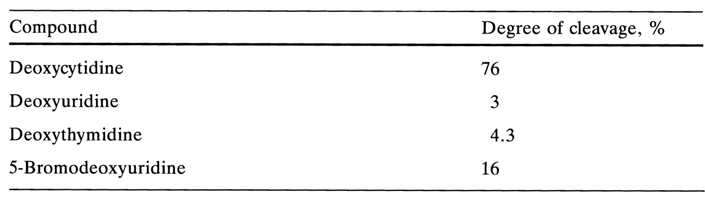
Introduction of a methyl group at position 5 of the pyrimidine ring does not produce any material effect on the hydrolysis rate (deoxythymidine is practically as stable as deoxyuridine). However, an electron-acceptor group at the same position makes cleavage of the glycosidic bond much easier: the 5-bromo derivative is hydrolysed more easily than the starting deoxyuridine (see above). The stability of the glycosidic bond in cytosine nucleosides is markedly affected by introduction of an acyl into the amino group of the heterocycle. For instance, 4-N-acyl derivatives of cytidine and deoxycytidine are hydrolysed in an acid medium much more readily than the starting nucleosides.
The glycosidic bond becomes even less stable in C5,C6-dihydropyrimidine nucleosides. The stability of such compounds in an acid medium is comparable to that of ordinary glycoside amines.
The lower stability of the glycosidic bond as a result of upset aromaticity of the pyrimidine ring is widely used while determining the structure of nucleosides. For example, to isolate the sugar or base from a pyrimidine nucleoside, it is first subjected to hydrogenation, then the 5,6-dihydronucleoside formed is hydrolysed under very mild conditions (0.1 N HCI, 1000C, 15 min).
2.4.2 Effect of Substituents in the Carbohydrate Moiety
The stability of the glycosidic bond is strongly dependent on the nature of the substituent at positions 2' and 3' in the carbohydrate moiety of the nucleoside. Ribonucleosides are much more stable (100-1000 times) toward hydrolysis than the corresponding deoxynucleosides (see above data on kinetics of hydrolysis).Substitution of an electronegative hydroxy group for hydrogen in the furanose cycle leads to a marked increase in stability of the glycosidic bond, as can be inferred from the following kinetic data (hydrolysis with 1 N HCI, 1000C):
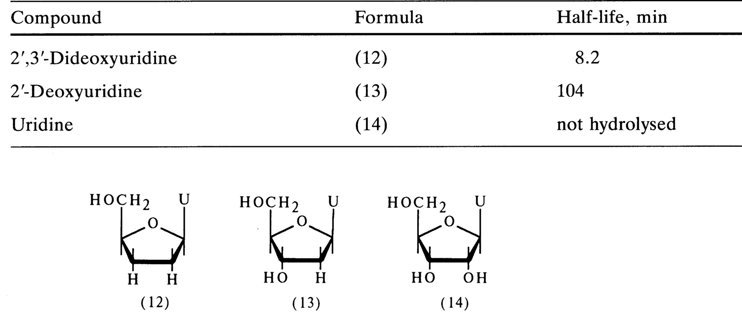
The glycosidic bond becomes even more stable when iodine is substituted for the hydroxy group or when such electron-acceptor groups as toluenesulfo- or 2,4-dinitrobenzoyls are inserted into the ribose.
2.4.3 Mechanism of Hydrolysis of N-Glycosidic Bonds
The linear relationship between the rate of acid hydrolysis of the N-glycosidic bond in nucleosides and the pH value of the reaction mixture suggests that the cleavage of this bond is preceded by protonation of the heterocyclic base, which, consequently, must be the step determining the rate of the reaction.
It is currently believed that hydrolysis of the N-glycosidic bond is not accompanied by opening of the furanose ring, as was previously assumed.
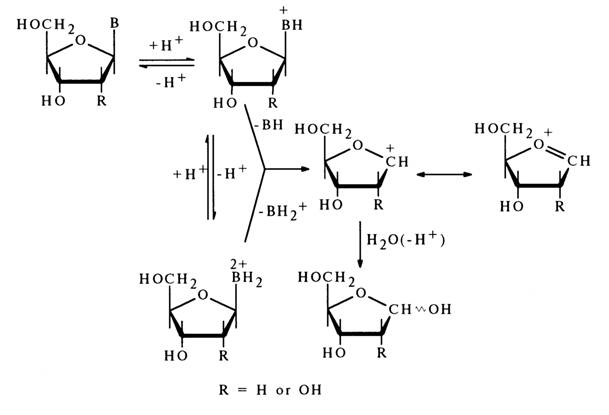
The above scheme shows clearly that in the course of the reaction, which seems to be based on a unimolecular mechanism, the base is detached from the carbohydrate moiety in a mono- or diprotonated state. For example, hydrolysis of the glycosidic bond in deoxycytidine may be written as follows:
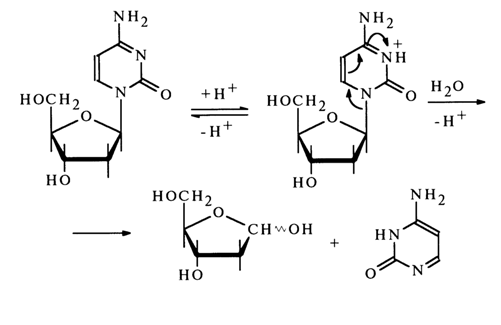
64 2 Properties of Nucleosides
The assumption is that during hydrolysis of deoxythymidine the base is protonated at the oxygen atom. For instance:
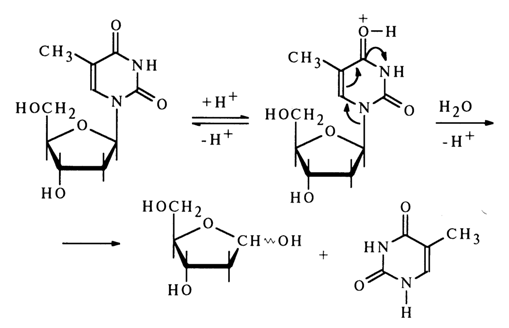
In the case of purine nucleosides, the protonation seems to proceed at the nitrogen of the imidazole ring, N7 , which (at least in guanine derivatives) is the site of maximum electron density.
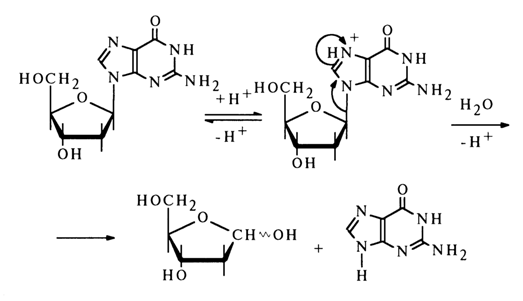
Such a mechanism for the cleavage of the N-glycosidic bonds in purines is further supported by the extremely high lability of nucleosides methylated at position 7 or 3. In this case, the positive charge at the respective nitrogen atom is fixed strongly enough. Therefore, such nucleosides are much less stable toward acid hydrolysis than the corresponding non-methylated analogues; moreover, they can also be hydrolysed in an alkaline medium. For example, the acid hydrolysis rate for 7-methyldeoxyguanosine is several orders of magnitudes above that for deoxyguanosine and is independent of the pH value of the reaction mixture. In addition this nucleoside is easily hydrolysed in an alkaline medium.
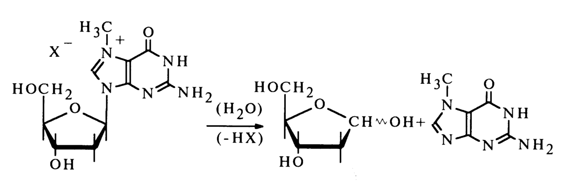
3-Methyldeoxyguanosine is even more labile in a weakly acid medium.
Thus, cleavage of the N-glycosidic bond in purine nucleosides usually involves conversion of at least one of the nitrogen atoms in the corresponding base into the onium form.
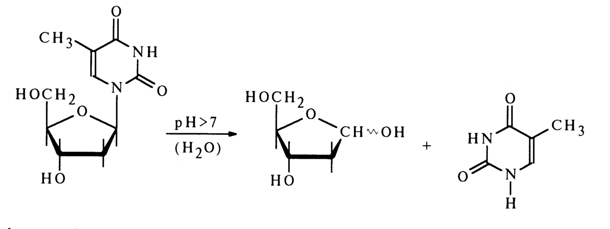
In conclusion, it should be pointed out that nucleosides are not always stable in neutral and alkaline media. There are some exceptions when the N-glycosidic bond is hydrolysed with relative ease in such media. For example, deoxythymidine, which is more or less stable in acid media, is hydrolysed rather rapidly at alkaline pH values close to neutral.
Adenosine and deoxyadenosine are hydrolysed, when heated with 1 N NaOH (1000C, 1 hour), by about 25 per cent to yield adenine, whereas guanine nucleosides are stable under the same conditions.
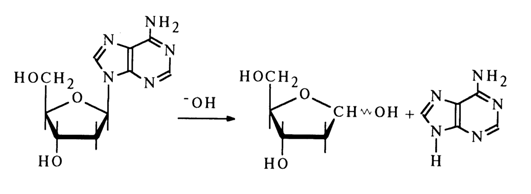
Notably, the pattern established for acid hydrolysis (in an acid medium guanosine nucleosides are hydrolysed more easily than adenosine ones) is not observed in this case. Unfortunately, the available experimental data are too scant to throw more light on the reasons for such difference.