![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
2.3 Reactions Involving Heterocyclic Bases and the Carbohydrate Moiety
A typical reaction in this category is the above-mentioned intramolecular alkylation of nucleosides, giving rise to a new ring, for example:
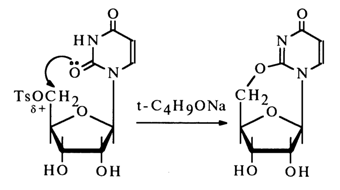
The starting compounds for obtaining cyclonucleosides may be aryl- or alkylsulfonates of nucleosides, containing both primary and secondary hydroxyl groups:
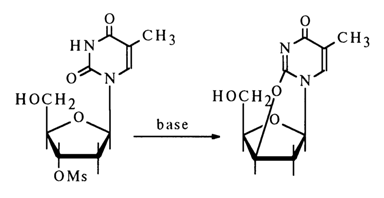
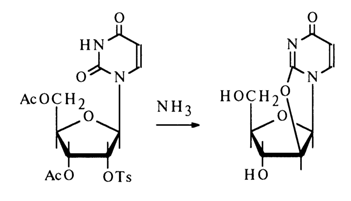
Similar transformations are also undergone by the corresponding 2'-derivatives of nucleosides:
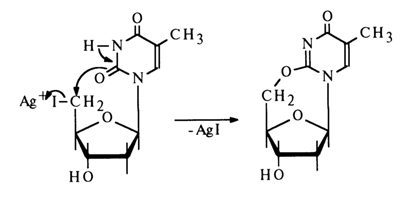
Uracil and thymine cyclonucleosides can also be derived from appropriate iododeoxynucleosides in the presence of silver salts:
Depending on the carbon atom (5', 3', or 2') of the carbohydrate moiety involved in the formation of the oxygen-containing bridge with the uracil (thymine) nucleus, distinction is made between O2, 5'-, O2, 3'- and O2, 2'-cyclic nucleosides. If we take a look at the ease of their formation, cyclic nucleosides can be arranged in the following order: O2, 2'-cyclic nucleoside > O2, 3'-cyclic nucleoside > O2, 5'-cyclic nucleoside.
Various cyclic nucleosides obtained from thymidine, uridine and 5-substituents of the latter are widely used in the synthesis of nucleoside analogues with a modified carbohydrate moiety and pyrimidine.
In addition to the intramolecular alkylation giving rise to new cyclic structures, other reactions have already been described, including those of alkylation and acylation, which involve both heterocyclic bases and the carbohydrate moiety.