![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
2.2 Reactions at the Carbohydrate Moiety
The pentose moiety of nucleosides is typically involved in three types of reactions: substitution for hydrogen atoms in hydroxyl groups, oxidation, and substitution for hydroxyl groups.
2.2.1 Substitution for Hydrogen Atoms in Hydroxyl Groups
This category includes such reactions as acylation and alkylation of sugars. They are used in the synthesis of nucleotides and their derivatives to block the hydroxyl groups of the carbohydrate moiety, therefore, this section deals primarily with the conditions under which the blocking groups are removed.
Acylation. Nucleosides react with anhydrides and acid chlorides in anhydrous pyridine at room temperature, giving nucleosides completely acylated in the carbohydrate moiety; for instance, acetylation may proceed as follows:
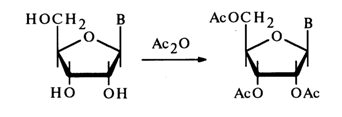
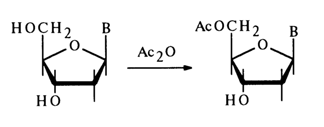
In the case of cytidine, the amino group of the base also undergoes acylation under the same conditions. The primary hydroxyl in deoxynucleosides lends itself more readily to acylation, which makes it possible to obtain 5'-monoacetyl derivatives under mild conditions:
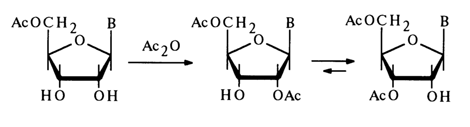
Acetylation of 5'-substituents of adenosine and uridine yields a mixture of 2',5'- and 3',5'-diacetyl derivatives. Heating of the latter with anhydrous pyridine or water brings about 2' -> 3' isomerization as a result of which 3',5'-di-O-acetylnucleoside is obtained with an almost quantitative yield:
Alkaline hydrolysis of O-acylnucleosides permits acyl groups to be removed under mild conditions.
The ester bond is also easily cleaved when O-acylnucleosides are treated with an alkaline solution of hydroxylamine:
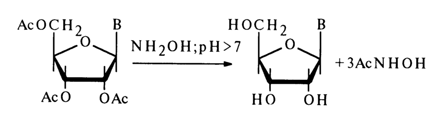
Aminoacylation of ribonucleosides has been studied most thoroughly. The synthesis on which all methods are based resides in a reaction of 5'-protected nucleosides with amino acids protected (or deprotected) at the amino group and activated at the carboxyl:
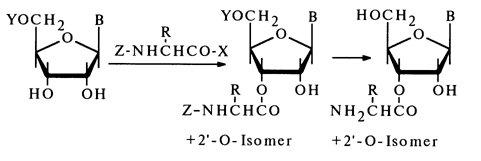
The methods for activating amino acids (the nature of X) and their protective groups (Z) have been borrowed from peptide synthesis; Y is usually a trityl group or its analogue.
An investigation of this reaction has led to two conclusions as regards the reactivity of nucleosides. Firstly, the activation of amino acids for the reaction with ribose hydroxyls must be sufficiently effective (only anhydrides and imidazolides of amino acids are capable of reacting). Secondly, in absolute solvents only the NH2 group of cytosine, if the hydroxyls of ribose are excepted, reacts with anhydrides, imidazolides and active esters of N-protected amino acids.
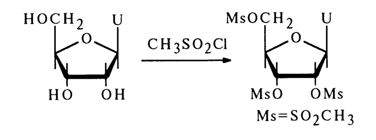
Similarly to the synthesis of 3'(2')-O-aminoacylnucleosides, the method based on the use of dicyclohexylcarbodiimide (the synthesis seems to involve formation of amino acid anhydrides as intermediates) makes it possible to introduce N-protected peptides into nucleosides. O-Aminoacyl-nucleosides can be isolated from these peptides by eliminating the protective groups. The hydroxyl groups of the carbohydrate moiety in nucleosides may also be acylated with derivatives of inorganic acids. Phosphorylation of nucleosides has become routine. Among the most widely used reactions of this type are also those with aryl(alkyl)sulfonyl chlorides, yielding esters of sulfonic acids.
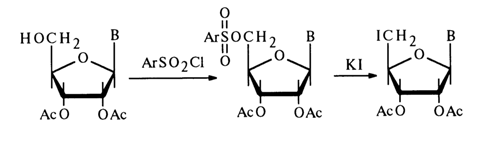
The arylsulfo group in such derivatives may be replaced by iodine. If a deprotected nucleoside is introduced into the reaction with aryl(alkyl)sulfonyl chloride, a nucleoside with a fully substituted ribose moiety can be obtained, for example:
Alkylation. As has already been mentioned, in addition to N-alkyl derivatives a reaction between alkylating agents (diazomethane, methyl iodide) and nucleosides also yields NO-dialkylnucleosides, the 2'-hydroxyl group undergoing alkylation with the greatest ease.
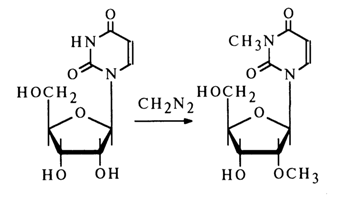
Selective 2'-0-methylation can be conducted successfully when adenosine or cytidine is heated together with diazomethane in an aqueous solution of 1,2dimethoxyethane. For example:
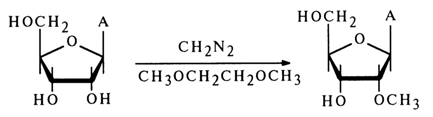
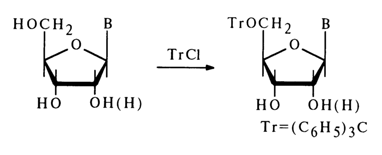
Alkylation of nucleosides with triphenylchloromethane proceeds in a selective manner, at the primary hydroxyl group.
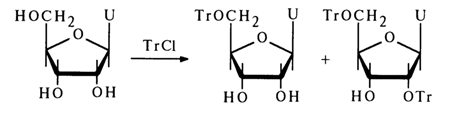
It is only in the case of uridine that 2',5,-O-ditrityluridine is obtained along with the 5'-O-monotrityl [5'-O-mono(triphenylmethyl)] derivative:
Triphenylmethyl esters are hydrolysed (elimination of the blocking trityl group) by the action of dilute hydrochloric or acetic acid. Incorporation of a methoxy group into the phenyl ring of trityl ester enhances the lability of the ether bond. The mono(methoxy)triphenylmethyl (MeOTr) group, for example, is completely removed in the presence of 80% acetic acid at 200C within two hours, whereas removal of the trityl group under the same conditions requires several days.
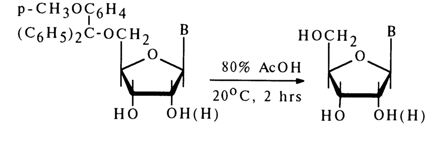
The reaction with para-mono(methoxy)triphenylchloromethane, MeOTrCl, is widely used in the synthesis of nucleoside derivates (especially in the synthesis of polynucleotides) by virtue of its high selectivity (only the primary hydroxyl group is involved) and the ease of removal of the protective group. Another rather popular reaction is acetal-yielding alkylation involving vinyl ethers.
In the first description of this reaction, uridine-3'5'-cyclic phosphate reacted with dihydropyrane:
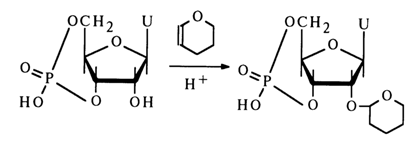
The reaction is conducted in organic solvents in the presence of dry hydrogen chloride. The tetrahydropyranyl groups is removed by mild acid hydrolysis.
Similar derivatives are yielded by reactions between nucleosides and 2methoxypropene:
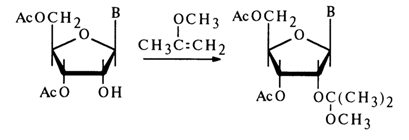
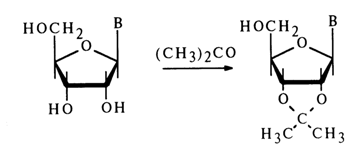
Reactions with Carbonyl Compounds. Ribonucleosides rather easily react with aldehydes and ketones in the presence of acid catalysts to yield cyclic acetals or ketals. The reaction with acetone is by far the most common:
In the case of benzaldehyde, a similar reaction gives rise to a new asymmetric center with the result that two diastereomers are formed:
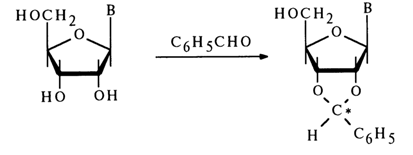
Ketals and acetals are easily hydrolysed in a weakly acid medium. The specificity of the interaction of aldehydes and ketones with the cis-glycol group and the ease of hydrolysis of the forming compounds are reasons why this reaction is extensively used for the selective protection of 2'- and 3'-hydroxyl groups in ribonucleosides.
It has already been mentioned above that nucleosides possessing a 2',3'-cis-glycol group (ribofuranosides) readily undergo oxidation with periodic acid (X is phosphoric acid, its ester or another group):
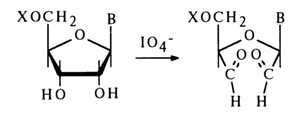
The reaction is rather specific and proceeds in an aqueous medium under extremely mild conditions. The oxidation products - dialdehydes - exist in aqueous solutions in the form of hydrates. Dialdehydes interact most readily with the usual reagents sensitive to aldehyde groups. For instance, their treatment with phenylhydrazine gives bis(phenylhydrazones):
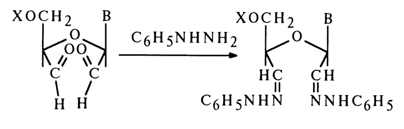
Dialdehydes and their derivatives are very easily oxidized in an alkaline medium with simultaneous cleavage of the glycosidic bond and detachment of XO~ (b-elimination) (here, X is phosphoric acid or its ester):
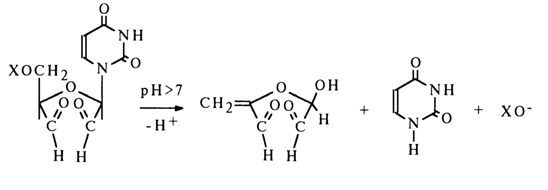
The products of periodate oxidation of ribonucleosides are readily reduced to the corresponding triols in the presence of sodium borohydride which is relatively stable in aqueous media:
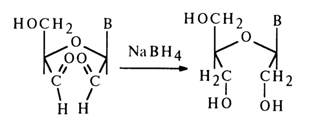
By using sodium borotritide, NaBT4, in this reaction one can easily label the oxidized nucleoside molecule.
The above-described oxidation reaction and its combination with reduction are widely used in various structural studies in the chemistry of nucleosides and nucleic acids.
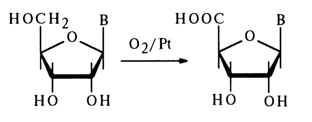
Treatment of ribonucleosides with oxygen over a platinum catalyst leads to selective oxidation of the primary hydroxyl group with formation of uronic acid derivatives: