![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
10.2 Tetrahymena Ribozyme
A lot more is known about the mechanism of catalytic action of the ribozyme isolated by T. Cech and coworkers from the intervening sequence RNA (IVS RNA) released during self-splicing of the 26S precursor of ribosomal RNA (pre-rRNA) from Tetrahymena. As shown in Figure 10-2, IVS RNA results
Fig. 10-2. Mechanism of formation of the IVS RNA ribozyme as a result of self-splicing of the Tetrahymena pre-rRNA. Straight lines - exons (mature rRNA sequences); wavy lines - IVS; circle - 5' splice site phosphate; square - 3' splice site phosphate; diamond - cyclization site phosphate (reproduced with permission from T. R. Cech, Science, 236, 1532-1539 (1987)).
from a sequence of transesterification reactions which begin with the phosphodiester bond between the 3' end of exon sequence and 5' end of the intron of the pre-rRNA being attacked by the 3' hydroxyl of guanosine or guanosine 5' phosphates. All these reactions proceed in vitro with intramolecular catalysis and without any proteins being present whatsoever. The end product of the reaction, IVS RNA, is an intron of pre-rRNA shortened from the 5' end by 19 nucleotides. It is this particular product that is the true enzyme involved in numerous experiments carried out to elucidate the mechanism of action of ribozymes of this class. At present, an intron shortened from the 5' end by 21 nucleotides in a feat of gene engineering is available. The structure of this region of pre-rRNA from Tetrahymena is shown in Figure 10-3.
Fig. 10-3. Model of secondary structure of IVS RNA. Letters stand for nucleotides highly conserved among group 1 introns. Also shown are the guanosine-binding site and the site at which pre-rRNA is attacked by guanosine during self-splicing (adapted from P. T. Flor et al., EMBO J., 8, 3391-3399 (1989)).
The IVS RNA contains ten double-helical regions on which P9 can be deleted without materially affecting the activity of the ribozyme. The short helical region P7 accomodates the site of specific binding of the guanosine which is involved in the first transesterification reaction during self-splicing (Fig. 10-2) and some other reactions catalysed by the IVS RNA. Most likely, a guanine base in the guanosine forms two hydrogen bonds with another G in the G264-C311 pair (Fig. 10-4).
There is convincing experimental proof of this binding pattern. Firstly, 1-methylguanosine and N2-methylguanosine are poor substrates for selfsplicing, whereas the bulky substituents at C8 and N9 of the guanine ring do not alter the substrate properties of guanosine. Secondly, the amino acid arginine is an effective competitive inhibitor of guanosine in these reactions. Thirdly, directed substitution of the A264-U311 base pair for G264-C311 results in the guanosine losing its substrate properties, but it can be effectively replaced by 2-aminopurine with citrulline, rather than arginine, acting as a competitive inhibitor of the latter (Fig. 10-5). The binding of the guanosine with the IVS RNA also seems to involve the 2'-OH group of ribose, in view of the fact that deoxyguanosine derivatives may compete with it only in high concentrations.
Fig. 10-4. Possible mechanism of binding of guanosine with the G264-C311 pair in IVS RNA. Also shown is pairing of 2-aminopurine with mutant explains why the binding of by arginine in the first case and by citrulline in the second (adapted from E Michel et al., Nature,342, 391-395 (1989)).
Fig. 10-5. Michel-Westhof model of the three-dimensional structure of the Tetrahymena group 1 intron. Black wriggled arrows indicate exons (reproduced with permission from F. Michel and E. Westhof, J. Mol. Biol., 216, 585-610 (1990)).
Then, for the first transesterification reaction to take place, the guanosine linked to G264 must be appropriately oriented with respect to the phosphorus in the phosphate group between the 3' end of exon and 5' end of intron. This is attained by fitting the IVS RNA into a specific tertiary structure whose hypothetical version is represented in Figure 10-5.
There is a good reason (the inversion of the configuration at the phosphorus) to believe that the nucleophile, 3' hydroxyl of guanosine, attacks the phosphorus atom by an in-line, SN2 (P) mechanism. It was shown by Cech and co-workers that the ribozyme-bound Mg2+ in the transition state is in direct contact with the pro-Sp oxygen of the phosphate group. This metal ion is coordinated to the 3' OH group of uridine to facilitate leaving of its 3'-oxyanion and to stabilize the trigonal bipyramidal transition state (Fig. 10-6). The stereochemistry of Mg2+ binding was evaluated by comparison of the activity of ribozymes after substitution of the phosphoryl group at the cleavage site to phosphorothioate group. Substitution of the pro-Sp oxygen atom with sulfur reduces the rate of the cleavage step more than 1,000-fold, whereas thio-substitution of the pro-Rp oxygen gives a small effect. The similar approach was used to prove the direct contact of the ribozyme-bound Mg2+ with 3' oxygen atom of the uridine residue at cleavage site: thio-substitution of this atom also dramatically reduces the ribozyme activity. It is important that this thio-effect was partially relieved when Mg2+ was replaced by Mn2+ which coordinates sulphur much stronger than Mg2+ does.
T. Steiz and J. Steiz suggested that a second ribozyme-bound Mg2+, ion can activate 3' hydroxyl of the guanosine for nucleophilic atack of the phosphoryl group. The two-metal-ion-mechanism they proposed (Fig. 10-6) could be common for other ribozymes and certain protein enzymes involved in phosphoryl transfer reactions.
Fig. 10-6. Cech-Steiz and Steiz mechanism of transition state stabilization by Tetrahymena ribozyme catalytic center. Hydrogen bonds and Mg2+-oxygen coordination are shown as dashed lines. Dotted P-O bonds indicate bonds partially formed or partially broken in the transition state (adapted from J. Piccirilli et al., Nature, 361, 85-88 (1993); T. Cech, in "The RNA World" (R. Gesteland and J. E Atkins, eds), pp. 239-269, CSH, 1993;T. A. Steiz and J. A. Steiz, Proc. Natl. Acad. Sci. USA, 90, 6498-6502 (1993)).
Fig. 10-7. Interaction of the internal guide sequence (IGS) of IVS RNA with the 5'splice site. It can be seen that base-pairing of IGS with the 5' splice site guides the guanosine attack of the phosphodiester bond between U and A (adapted form F. L. Murphy and T. R. Cech, Proc. Natl. Acad. Sci. USA, 86, 9218-9222 (1989)).
Notably, both 5' and 3' splice site phosphodiester bonds of the Tetrahymena 26S pre-rRNA are hydrolysed at a much faster rate than average phosphodiester bonds. There is reason to believe that their hydrolysis as well as the specific hydrolysis of the cyclic IVS shown in Figure 10-2 are based on the same general mechanism as the transesterification involving the guanosine, since the phosphate group also remains at the 5' end of the polynucleotide chain. A similar mechanism of hydrolysis must be involved in the cleavage of pre-tRNA by ribonuclease P.
Another important functional site in the IVS RNA of Tetrahymena is the GGGAGG sequence termed "internal guide sequence" (IGS), which occupies the 3' end of Pl. During self-splicing it participates in the complementary pairing with the 5' splice site (Fig. 10-7). The formation of a non-canonical U-G base pair immediately preceding the phosphate group attacked by the guanosine is highly important for the reaction. It may be substituted only by another non-Watson-Crick pair.
In spite of the fact that the IGS is substantially screened by the ribozyme structure, it can be paired with an erogenous RNA containing a region with a nucleotide sequence complementary to the IGS. In the presence of guanosine and magnesium ions this RNA undergoes specific cleavage (Fig. 10-8). By substituting nucleotides in the IGS one can alter the specificity of the ribozyme. For example, by varying the trinucleotide sequence immediately adjacent to the G-U pair in the IGS one can create 64 ribozymes specifically cleaving respective regions of the RNA. They only differ in the magnesium ion concentration optimal for the transesterification reaction, due to differences in the stability of ribozyme-substrate duplexes. By analogy with DNA restriction enzymes these ribozymes have been termed RNA restriction endonucleases.
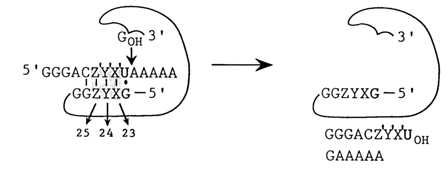
Fig. 10-8. Construction of RNA restriction endonucleases on the bases of IVS RNA. Note that the 5' splice site is replaced by an exogenous RNA. The XYZ sequence varies in such a manner as to retain the complementarity of the Z'YX' sequence (adapted from E L. Murphy and T R. Cech, Proc. Natl. Acad. Sci. USA, 86, 9218-9222 (1989)).
The binding of an erogenous RNA with the IGS in the ribozyme is a rather complex process. In any event, both the substrate and the reaction product are bound with the ribozyme, the binding being about 104 times stronger than might have been expected had the process confined itself to formation of an RNA-RNA duplex only. Apparently, this binding also involves tertiary interactions.
Interestingly, the pre-rRNA self-splicing process considered here has turned out to be reversible. If, after the splicing is over, excess RNA with a sequence complementary to the IGS is added to the IVS RNA with an additional G at its 5' end (L-19 IVS RNA), the ribozyme will be incorporated into the substrate RNA as shown in Figure 10-9. And if a ribozyme with IGS at the 5' end is used, linked to its 3' end will be only the 3'-terminal portion of the cleaved substrate. This reaction provided the basis for an interesting method for selective amplification of ribozymes capable of cleaving the nucleotide sequences of interest. Its principle is illustrated in Figure 10-10. The key point here is the fact that the amplification is triggered by a cleavage reaction. Consequently, if a wild ribozyme is replaced by a set of its mutants, it is possible to choose one that will cleave the desired substrate. This is precisely how a ribozyme cleaving single-stranded polydeoxyribonucleotides rather effectively has been selected.
Along with specific cleavage of RNA, ribozymes produced from the IVS RNA may also catalyze a number of important biochemical reactions. It has been found that the substrates for it may include not only phosphodiesters but also -monoesters. For example, the ribozyme may transfer a phosphate (i.e., act as phosphotransferase) reversibly from the 3' end of the oligo-Cp substrate to that of its own chain. The optimal pH value for the reaction is 5 - that is, the ribozyme exhibits higher activity with respect to the monoanionic form of the 3'-phosphate, as compared to the dianion. At pH 4-5, the phosphoribozyme is hydrolysed with liberation of an inorganic phosphate or, in other words, the ribozymes display acid phosphatase activity.
Fig. 10-9. Reverse splicing of the Tetrahymena group I intron (compare with the direct splicing illustrated in Fig. 10-2). The diamond denotes the 3' splice phosphate that becomes the ligation junction phosphate. The releasing G, which initially was at the 5' end of IVS RNA, is shown in italics (adapted from S. A. Woodson and T R. Cech, Cell. 57. 335-345 (1989)).
Fig. 10-10. Selective amplification of L-21 IVS RNA. Note that the process is based, firstly, on the ribozyme capacity for reversible self-splicing (compare with the first step of the reaction shown in Fig. 10-9) and, secondly, on the fact that only ribozymes capable for transesterification (i.e. active ribozymes) can be amplified. An important step of this process is also the formation of a double-helical DNA containing a T7 RNA polymerase promoter (adapted from D. L. Robertson and G. F. Joyce, Nature, 334, 467-468 (1990)).
The guanyl residue, at the 3' end of the ribozyme may also participate in a nucleotidyl transferase reaction which, in the final analysis, results in extension of the oligonucleotide chain (Fig. 10-11).
The IVS RNA ribozyme may also catalyze transesterification reactions between two dinucleotides:
in which case its substrate may only be a dinucleotide with a natural 3', 5'-phosphodiester bond.
Fig. 10-11. Mechanism of the nucleotidyltransferase reaction catalysed by L-19 IVS RNA (adapted from A. L. Zaug and T R. Cech, Science, 231, 470-475 (1986)).
These reactions can be regarded as simplest instances of enzymatic polymerization of ribonucleotides, although the length of the product is limited to about 15 nucleotides.
However, the IVS RNA can be converted into a ribozyme with an activity similar to that of RNA replicase, which is capable of catalysing template-directed RNA polymerization on a template not linked to the ribozyme. To this end, the ribozyme is synthesized in vitro in the form of two parts one of which is a P1-like stem-loop element and the other comprises the rest of the ribozyme slightly shortened from the 3' end. Thus, the IGS becomes separated from the main part of the ribozyme.
The truncated ribozyme may catalyze (albeit with a much higher kcat than in the case of normal self-splicing) specific cleavage of P1 at the usual site in the presence of guanosine (Fig. 10-12). What is more important, it has been found to be capable of ligating two oligonucleotides in P1-like structures shown in Figure 10-12.
In the presence of spermidine, the polyamine stabilizing the RNA secondary structure, the ribozyme catalysed ligation of oligonucleotides in structures with any Watson-Crick or wobble base pair at the ligation junction (right substrate). It is also shown that the presence of a loop in the P1 element is of no importance for the transesterification reaction. This allowed the ligation of oligonucleotides catalysed by the ribozyme to be conducted on linear templates (Fig. 10-13).
Fig. 10-12. Ligation reaction catalysed truncated IVS ribozyme (deprived of PI, P9.1 and P9.2 elements) (adapted from J. A. Doudna and J. W Szostak, Nature, 339, 519-522, (1989)).
Fig. 10-13. Multiple oligonucleotide ligation catalyzed by the truncated ribozyme (adapted from J. A. Doudna and J. W. Szostak, Nature. 339, 519-522 (1989)).
The Tetrahymena IVS has turned out to be the first representative of a large group of introns (usually referred to as group 1 and la introns) similar to it in terms of secondary and tertiary structure. Their guanosine-binding centers are also similar. They have been discovered in a great variety of organisms, including fungal mitochondria and chloroplasts as well as such evolutionarily distant representatives of procaryotes as bacteriophages. Most of them have been found to exhibit enzymatic activity in vitro.