![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
1.3 Carbohydrate Moieties of Nucleosides
The nucleosides isolated from nucleic acids contain only two simple sugars, D-ribose and 2-deoxy-D-ribose, which, as has already been mentioned, determine the nucleic acid type (RNA and DNA).
As far back as 1891, Albrecht Kossel pointed out that hydrolysis of RNA (this name was non-existent at that time) yielded a carbohydrate which was isolated in a crystalline state 18 years later and identified as a yet unknown sugar D-ribose. When oxidized under certain conditions, this simple sugar underwent conversion first into D-ribonic acid and then into optically inactive trihydroxyglutaric acid.
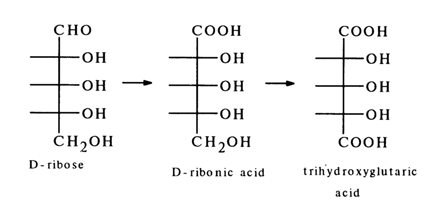
The structure of D-ribose was confirmed by its synthesis. Synthetic D-ribose turned out to be identical with the crystalline simple sugar isolated from RNA nucleosides.
Serious difficulties had to be overcome in establishing the nature of the sugar contained in the nucleosides that are the building blocks of DNA (i. e. deoxyribonucleosides). It was found that the monosaccharide was more labile than D-ribose. Another finding was that it formed a hydrazone readily soluble in water, but no osazone, which rendered isolation of its hydrolysates difficult. It was long believed that this sugar was a hexose because hydrolysis of nucleosides isolated from DNA yielded levulinic acid. In 1929, however, it became possible to isolate, through mild hydrolysis, a crystalline carbohydrate from the guanine nucleoside of DNA, which turned out to be deoxypentose. Its structure was established beyond any doubt after the synthesis of 2-deoxy-Lribose. Both preparations exhibited similar properties as well as identical absolute, but opposite in sign, values of optical rotation. The conclusion that followed was that the sugar found in deoxyribonucleosides was 2-deoxy-D-ribose.
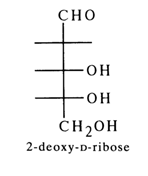
It was also established that mild acid hydrolysis of DNA in the presence of benzyl mercaptan yielded benzyl mercaptal of 2-deoXY-D-ribose, which can be isolated in crystalline form. As was demonstrated in the fifties by chromatographic analysis, the only sugar in DNAs of plant, animal and bacterial origin is 2-deOXY-D-ribose. In 1954, a full description of 2-deoXY-D-ribose isolated from different types of DNA was provided by comparison with its synthetic analogue (melting point of mixed sample, optical rotation, synthesis of derivatives of known structure).
In the late fifties, an unusual carbohydrate was found in some preparations of RNA extracted from plants and animals, which was identified as 2-O-methyl-D-ribose.
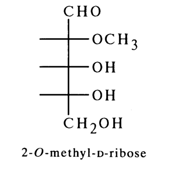
As was established later, 2-O-methyl-D-ribose is present in very small amounts in certain RNAs, which is why this sugar is referred to as a rare or minor component of RNA.