![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
1.2 Pyrimidine and Purine Bases
Pyrimidines and purines, first isolated from hydrolysates of nucleic acids (1874-1900), were identified using classical methods of organic chemistry (see Table 1-1). An important contribution was made by Emil Fischer who must be credited with the earliest synthesis of purines (1897). Today, pyrimidines and purines can be identified rapidly and reliably by chromatographic and spectrophotometric techniques.
Pyrimidines, also known as meta-diazines, are structurally akin to benzene and pyridine. Pinner (1885), who had noticed this analogy, coined a new term from the words "pyridine" and "amidine", emphasizing thereby that apart from aromaticity pyrimidines also exhibit properties inherent in amidines. Pyrimidines are numbered according to the Chemical Abstracts Service registry.
Given below are the formulas of the pyrimidines whose compounds are nucleic acid constituents, such as uracil, thymine, and cytosine (see Table 1-1).
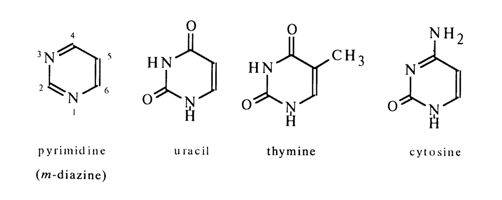
Thymines and cytosines are usually present in DNA, while those of uracil and cytosine are found in RNA. The DNAs of some phages are exceptional (see Table 1-4) in that they contain 5-hydroxymethylcytosine or its glycosides associated with the 5-hydroxymethyl group instead of cytosine (even-numbered T phages) and 5-hydroxymethyluracil (phage SP8) or uracil (phage PBS1) instead of thymine.
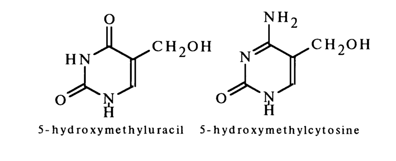
Uracil, thymine, and cytosine are ubiquitously present in nucleic acids in the form of the corresponding nucleosides in significant amounts (each accounting for at least 5 % of the total bases in nucleic acids).
These compounds are usually referred to as the major pyrimidine bases of nucleic acids. In certain DNA and RNA species, some uracil and cytosine derivatives (usually N-alkyl ones) have been found. Such pyrimidine bases are referred to as rare or minor. Their structural formulas (as part of the corresponding nucleosides) are given in Tables 1-5 and 1-6. Minor pyrimidine bases do not occur in all nucleic acids. and the content of each base is usually below one or two per cent.
Purines are heterocyclic systems consisting of a pyrimidine and an imidazole condensed at the 4-5 bond. The term "purine" (from "purum" and "uricum") was introduced in 1898 by Emil Fischer. Purines are also numbered according to the Chemical Abstracts Service registry.
Both DNA and RNA contain two major purine substituents - adenine and guanine:
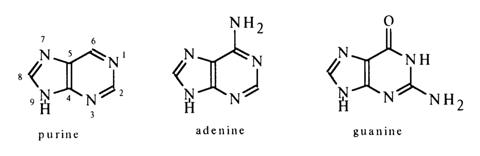
Minor purines differing from adenine or guanine by the presence of alkyl (more commonly, methyl), acyl and other groups have also been isolated from some nucleic acids. The structural formulas of the minor purines present in nucleosides are presented in Tables 1-5 and 1-6.
1.2.3 Nomenclature of Pyrimidines and Purines
In the case of natural pyrimidines and purines, the above-mentioned common names are widely used. As regards synthetic bases and various analogues or modifications of natural pyrimidines and purines, specialists resort to the nomenclature usually applicable to heterocyclic bases, with appropriate numbering of atoms in the pyrimidine or purine ring. For example, thymine is called 2,4-dioxo-5-methyltetrahydropyrimidine or 2,4-dihydroxy-5-methylpyrimidine, whereas guanine is called 2-amino-6-oxodihydropurine or 2-amino-6-hydroxypurine.
Since the plane of symmetry in unsubstituted pyrimidine passes through C2 and C5, positions 4 and 6 are equivalent.
Bases are very often designated by abbreviations (symbols) when writing structural formulas of nucleosides and their derivatives. Table 1-1 lists the names and symbols of the major pyrimidine and purine bases constituting RNA and DNA.
Table 1-1. Names and Abbreviated Symbols of Major Pyrimidines and Purines Constituting Nucleic Acids
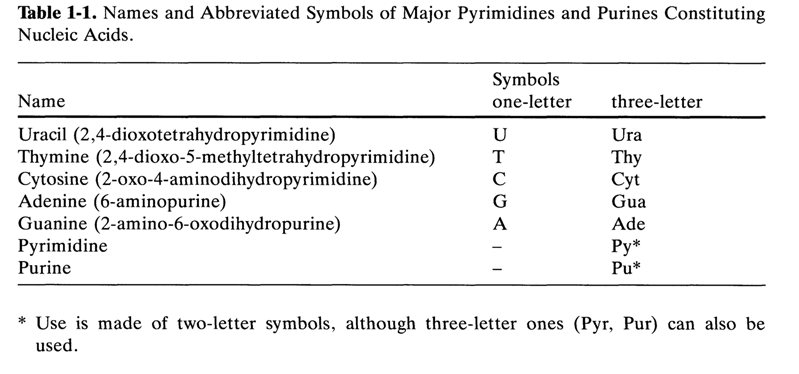
The symbolic notation of the formulas in this and other chapters follows the recommendations of the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Biochemistry (IUB) and has been approved by the 3rd All-Union Working Session on Nucleotide Chemistry.