![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
1.4 Bonding Between Carbohydrate Moiety and Heterocyclic Base
It is logical to assume that adenines and guanines in nucleosides are linked to the carbohydrate moiety in the same manner. This is why bonding at the oxygen atom is impossible in the case of guanine. The ribosides of adenine and guanine readily lend themselves to deamination in the presence of nitrous acid with the linkage between the base and carbohydrate remaining intact.
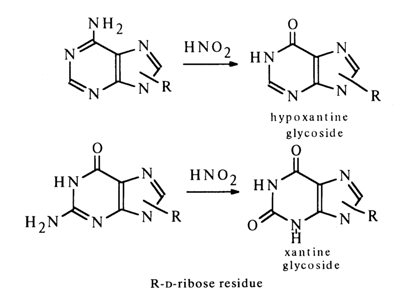
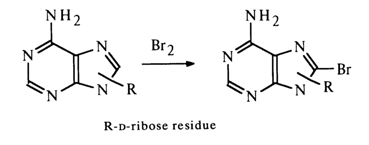
Consequently, the possibility of bonding at amino groups is also ruled out. Thus, the sugar moiety may be linked to adenines and guanines at C8 (C-C bond) or at one of the nitrogens (C-N bond) of the pyrimidine (N1 or N3) or imidazole (N7 or N9) rings. These two types of bonds must differ markedly in stability under conditions of acid hydrolysis, since it is known that N-glycosides undergo hydrolysis rather easily, whereas C-glycosides are extremely stable. The ease of hydrolysis of purine nucleosides attests to their being N-rather than C-glycosides. This is also corroborated by the fact that the adenine and guanine nucleosides can be converted into C8-substituted derivatives (the substituent at the carbon in the imidazole ring is absent). For example, adenine riboside is readily brominated at the imidazole ring carbon:
out of the four nitrogens of the purine base those in the pyrimidine ring
(N1 and N3
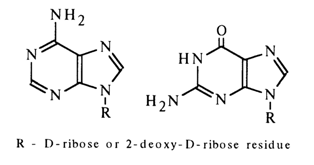
) are excluded because methylation of the xanthine riboside yielded, as has already been mentioned, by deamination of guanine riboside gives theophylline (1,3-dimethyl xanthine) riboside.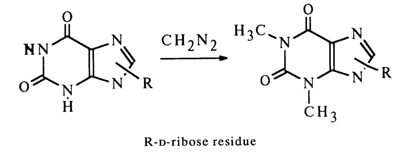
The nitrogens at positions 7 and 9 can be regarded as possible binding sites.
The question as regards the site of sugar to base binding was answered once and for all by Gulland in the forties. He was the first to take advantage of the fact that the UV spectra of the purine base containing an alkyl radical and a carbohydrate moiety at the same nitrogen atom are identical and markedly dependent on the position of the substituted nitrogen in the heterocyclic nucleus. Comparison of the UV spectra of adenine riboside with those of 7- and 9-methyladenine showed that the adenine nucleoside has a UV spectrum that is virtually identical with that of 9-rnethyladenine and bears no resemblance to that of 7-methyladenine.
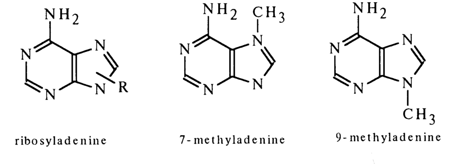
Similarly, the UV spectrum of guanine riboside looks like the spectrum of 9-methylguanine and differs from that of 7-methylguanine. These findings clearly indicate that purine nucleosides are 9-ribosylpurines. The structures of adenine and guanine deoxyribosides are very much the same.
Rigorous proof that the sugar moiety in purine nucleosides is at position 9 was provided by the synthesis of the respective nucleosides, conducted by Todd and coworkers.
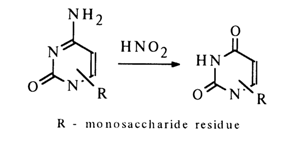
In the case of pyrimidine nucleosides, the involvement of the substituent at position 4 in the glycosidic bond should be ruled out in view of the fact that deamination of ribosylcytosine may yield ribosyluracil:
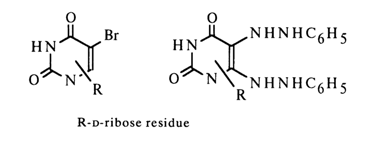
The greater stability of pyrimidine nucleosides during acid hydrolysis, as compared to O-glycosides, suggests that the oxygen at position 2 does not participate in the formation of a bond with the carbohydrate moiety either. The same applies to the carbons at positions 5 and 6, since the substitution products associated with C5 and C6 [5-bromo- and 5,6-di(phenylhydrazyl)-nucleosides] have been derived from a natural uracil nucleoside.
Consequently, only Nl and N3 of the pyrimidine ring can be linked to the sugar. Methylation of ribosyluracil gives mono-N-methylribosyluracil (uracil is converted into 1,3-dimethyluracil under the same conditions) which breaks down to 3-methyluracil during acid hydrolysis:
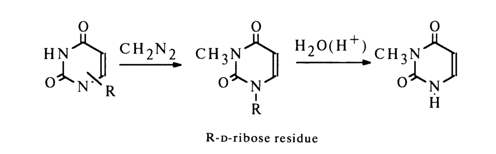
This is indicative of the fact that uracil nucleoside, just like cytosine nucleoside, is 1-riboside.
The similarity between the UV spectra of cytosine riboside and deoxyriboside, as well as the isolation of 3-methylthymine from DNA methylation products. attest to linkage between the sugar of deoxynucleosides and N1 of the pyrimidine ring.
The conclusions as to the site of ribose binding in nucleosides have been corroborated by X-ray structure analysis.