![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
1 Structure of Nucleosides
1.1 Introduction
The term "nucleoside" was introduced in 1909 by Levene and Jacobs to denote carbohydrate derivatives of the purine bases isolated from yeast nucleic acid hydrolysates. Later, the term was expanded to additionally cover compounds containing pyrimidines and other heterocyclic bases. Today, it is applied to a broad class of naturally occurring and synthetic compounds that are essentially N- and C-glycosides - derivatives of various carbohydrates and heterocyclic compounds.
Nucleic acids contain nucleosides of two types: derivatives of D-ribose, known as ribonucleosides, and those of 2-deoxy-D-ribose, known as deoxyribonucleosides or, sometimes, deoxynucleosides.
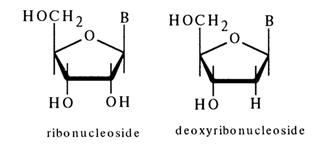
Such classification of nucleosides stems from the nature of their
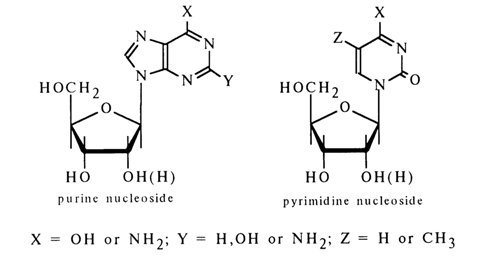
constituent sugar. According to the structure of the heterocyclic base (B), which is the
other component of nucleosides, the latter are also divided into pyrimidine and purine
bases (see Tables 1-1, 1-2,
1-3 and 1-4) ![]() .
.
Nucleosides can be isolated from nucleic acids (NA) after chemical or enzymatic hydrolysis. Ribonucleic acids (RNA) are hydrolysed to nucleosides when boiled with aqueous pyridine, with a diluted ammonia solution or in an ammonium formiate buffer at pH 4.0. Nucleotides are yielded as intermediates:
® Ribonucleotides ® RibonucleosidesRNA
Deoxynucleosides are produced by the enzymatic hydrolysis of deoxyribonucleic acids (chemical hydrolysis being accompanied by some side processes). To this end, use is normally made of snake venom containing the enzymes phosphodi- and phosphomonoesterase.
Some nucleosides occur in a free state and can be isolated by direct extraction.
The isolation and identification of nucleosides usually involve ion-exchange, thin-layer and gas-liquid chromatography as well as UV spectroscopy and mass spectrometry.
Acid hydrolysis of the nucleosides present in nucleic acids yields a heterocyclic (pyrimidine or purine) base and pentose. Nucleosides do not react at the aldehyde group, the implication being that the linkage between the sugar and bases is via a glycosidic bond. To establish the structure of each nucleoside present in a nucleic acid one must determine: (1) the structure of the constituent base; (2) the structure of the constituent sugar; (3) the type of the bond linking the two (the site at which the sugar is attached to the base); (4) the size of the oxide ring in the carbohydrate moiety; and (5) the configuration of the glycosyl (anomeric) moiety.