![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
4.7 Hydrolysis of N-Glycosidic Bonds
The N-glycosidic bonds in nucleotides, as well as nucleosides, are rather stable at neutral and alkaline pH values, but prone to acid hydrolysis. The N-glycosidic bonds in purine nucleotides are hydrolysed with much greater ease than in pyrimidine ones.
The mechanism of acid hydrolysis of N-glycosidic bonds and the influence of various factors on their stability were discussed at length in Chapter 2. The described regularities apply to nucleotides as well. This section deals only with the effect of the phosphate group on the stability of N-glycosidic bonds. The rates of acid hydrolysis of N-glycosidic bonds in purine nucleotides and the corresponding nucleosides differ but insignificantly, especially in the series of adenine derivatives (pH 1.0, 370C):
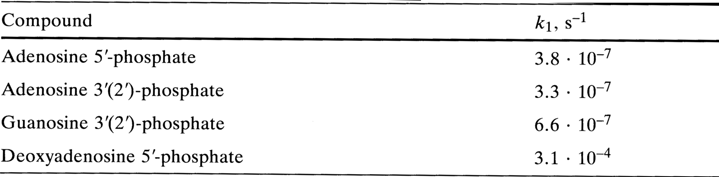
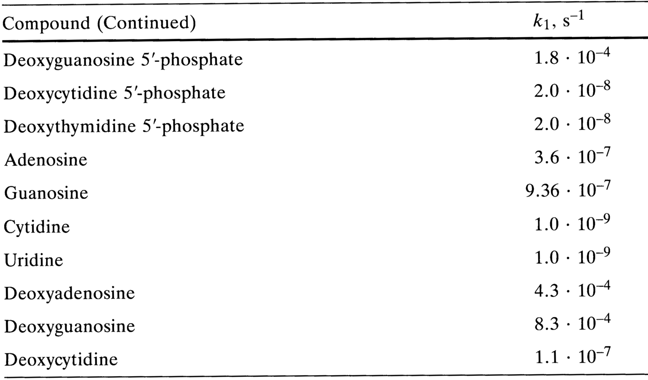
Similarly, minor differences in hydrolysis rate are observed in the case of adenosine 3'(2')-phosphate and guanosine 3'(2')-phosphate as well as the corresponding nucleosides in 6 N hydrochloric acid at 1000 C.
As regards pyrimidine deoxyribo derivatives, incorporation of the phosphate group into a nucleoside renders the glycosidic bond more stable. This stability increases from mono- to diphosphates (0.4 N H2S04, 1000 C):
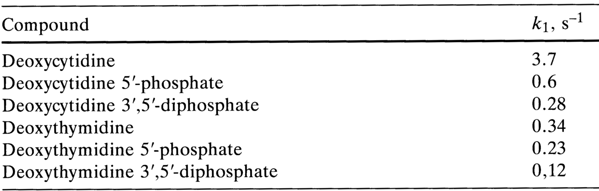
This trend seems to stem from the fact that undissociated (under conditions of acid hydrolysis) phosphate groups are strong electron-acceptor substituents because their phosphorus atom carries a partial positive charge. The effect of the phosphate group on the stability of the glycosidic bond is comparable with that of the toluenesulfo or 2,4-dinitrobenzoyl group.
An opposite effect on the glycosidic bond stability in uridine derivatives is exerted by the 3',5'-cyclic phosphate group. Uridine 3',5'-eyclic phosphate loses its base very rapidly when treated with 1 N hydrochloric acid (the half-life at 1000 C being only 8 min). Uridine 5'- and 3'-phosphates are rather stable under such conditions. Similar lability is displayed by other pyrimidine nueleoside 3',5'-cyclic phosphates as well.
In contrast, the N-glycosidic bonds in purine ribonucleoside 3',5'-cyclic phosphates are hydrolysed at a much slower rate than in the corresponding monophosphates.
The marked labilizytion of the N-glycosidic bonds in pyrimidine nucleoside 3',5'-cyclic phosphates and, on the other hand, their increasing stability in analogous purine derivatives (as compared to ribonucleoside 5'- and 3'-phosphates) are most likely due to the special conformation of pentose in their molecules.