![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
3.3 Structure of Nucleotides
It has already been pointed out that nucleotides, which are essentially monomer units of nucleic acids, represent nucleosides monophosphorylated at the sugar moiety. Since the structure of nucleosides was discussed in the preceding chapter, here we shall deal only with those aspects of nucleotide structure which have to do with the position of the phosphate group in their molecule.
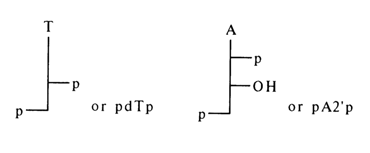
Nucleoside 5'-phosphates result from hydrolysis of RNA and DNA in the presence of the enzyme phosphodiesterase (PDE) isolated from snake venom as well as from actinomyces and yeasts.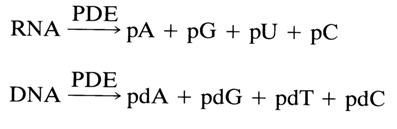
Nucleotides of this type were isolated for the first time from muscle extracts rather than nucleic acid. As far back as 1847, Liebig isolated a nucleotide which was named muscle inosinic acid. This nucleotide seems to result from enzymatic deamination of 5'-adenylic acid (adenosine 5'-phosphate). The latter is known to be present in muscles in a free state as an intermediate product of biosynthesis.
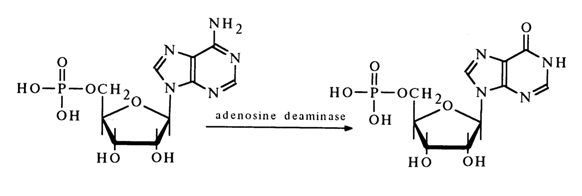
As has been established in recent years, inosinic acid in the form of phosphate of a minor nucleoside, inosine to be exact (see Table 1-5), is present in some RNAs and plays an important role in their functioning.
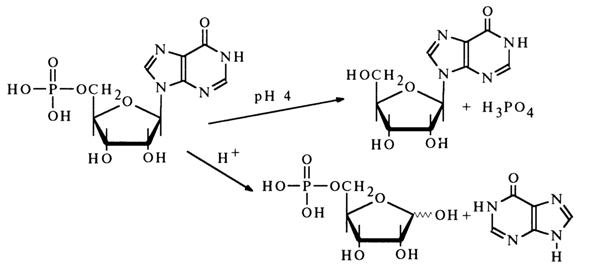
In spite of the fact that inosinic acid was isolated long before the discovery of nucleic acids, its structure was established only in 1909 when it was demonstrated that this nucleotide is hydrolysed in a weakly acid medium to inosine and phosphoric acid, whereas its acid hydrolysis yields ribose 5-phosphate and hypoxanthine:
The structure of ribose 5-phosphate was established by converting it, during oxidation with dilute nitric acid, into 5-phospho-D-ribonic acid (l):
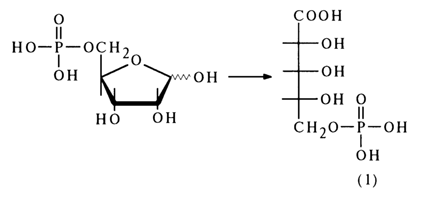
If the phosphate group in the ribose phosphate under investigation had been linked to the 2- or 3-hydroxyl group of ribose, the oxidation would have resulted in phosphotrihydroxyglutaric acid (2):
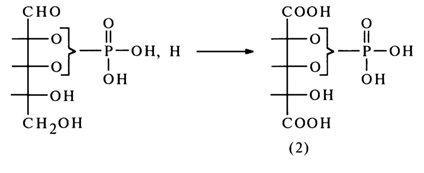
However, this acid has never been found among the oxidation products of the corresponding ribose phosphates.
Ribose 5-phosphate was then converted into a methyl glycoside whose reduction produced an optically active ribite 5-phosphate:
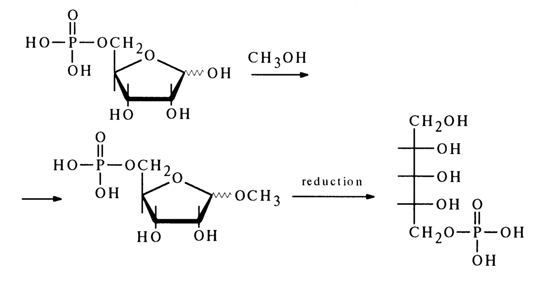
Final evidence to support the structure of ribose 5-phosphate was provided by its synthesis from 2,3-isopropylidene-1-O-methylribofuranoside according to the following scheme:
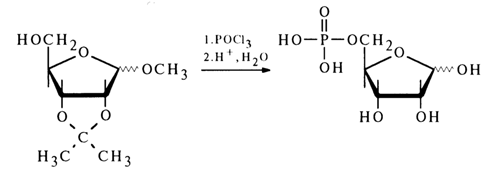
The ribose phosphate isolated after hydrolysis of inosinic acid and the synthesized ribose 5-phosphate turned out to be identical. This finding has suggested that the phosphate group in inosinic acid and, consequently, in muscle adenylic acid, occupies position 5' in the corresponding nucleoside. The adenosine phosphate isolated from RNA after its hydrolysis with snake venom PDE was found to be identical with muscle adenylic acid or, in other words, adenosine 5'-phosphate. Its structure was also corroborated by synthesis from 2', 3'-O-isopropylideneadenosine:
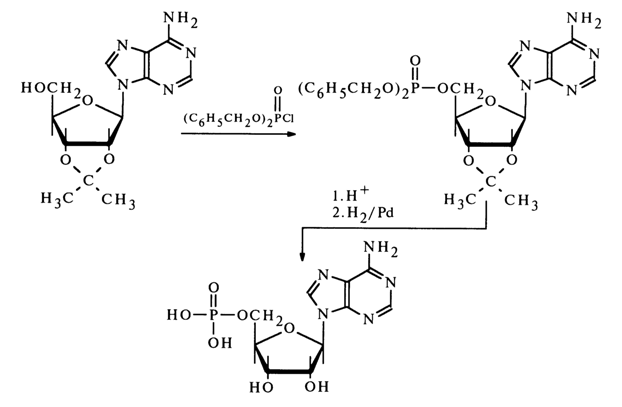
The structure of other ribonucleoside 5'-phosphates (pG, pU and pC), formed during PDE hydrolysis of RNA has been established in a similar manner and supported by syntheses from guanosine, cytidine and uridine derivatives, conducted using the above procedure. It should be noted that the phosphorylating agent in the synthesis of guanosine 5'-phosphate was tetra-para-nitrophenyl pyrophosphate:
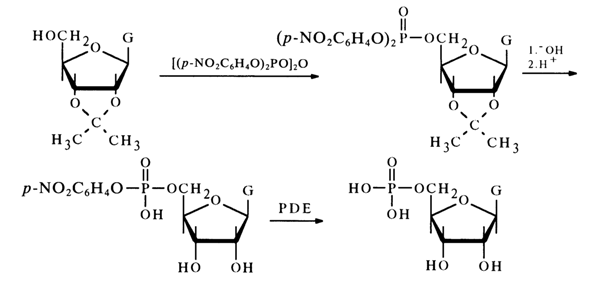
During mild alkaline hydrolysis of a completely protected nucleoside, one of the para-nitrophenyl groups was removed; the isopropylidene group was removed as usual by acid treatment. The other para-nitrophenyl group was removed by PDE hydrolysis. Comparison of each of the synthesized nucleoside 5'-phosphates (pA, pG, pC and pU) with the nucleotides isolated from RNA has shown them to be identical with each other.
Ribonucleoside 5-phosphates with a free cis-glycol group (as opposed to nucleoside 2'- and 3'-phosphates) are easily oxidized with periodic acid:
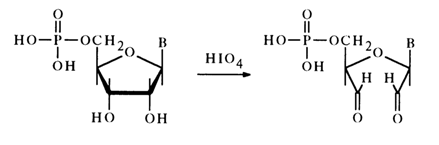
The oxidation requires 1 mole of periodic acid, without formation of formic acid in the process.
The results of oxidation with periodic acid have confirmed the structure of the ribonucleoside 5'-phosphates isolated from RNA. Natural ribonucleoside 3'(2')-phosphates are not oxidized under these conditions. It is for this reason that the periodate oxidation method is widely used to determine the position of the phosphate group in ribonucleoside phosphates.
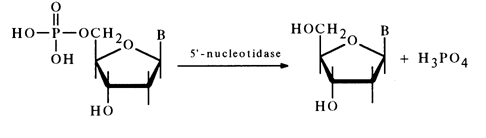
Synthetic and naturally occurring ribonucleoside 5'-phosphates are easily dephosphorylated in the presence of snake venom PME also known as 5'nucleotidase:
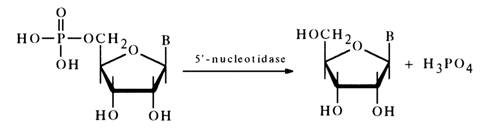
At present, in order to determine the position of the phosphate group
in nucleotides of unknown structure extensive use is made of this enzyme because it does
not break phosphomonoester bonds in nucleoside 3'- and 2'-phosphates. The ability of a
nucleotide to break down to a nucleoside and phosphoric acid under the action of
5'-nucleotidase is considered to be solid proof that its phosphate group is at position
5'. The crystalline deoxyribonucleotides isolated in 1935 during enzymatic ![]() hydrolysis of DNA also turned out to be
5'-phosphates. The determination of the phosphate group position in these compounds was
based on the fact that their breakdown under the effect of 5'-nucleotidase resulted in
equimolar quantities of the corresponding deoxynucleosides and phosphate:
hydrolysis of DNA also turned out to be
5'-phosphates. The determination of the phosphate group position in these compounds was
based on the fact that their breakdown under the effect of 5'-nucleotidase resulted in
equimolar quantities of the corresponding deoxynucleosides and phosphate:
The structure of the deoxynucleoside 5-phosphates derived from DNA was corroborated by synthesis. Given below by way of example is the synthesis of deoxythymidine 5'-phosphate from 3'-O-acetyldeoxythymidine obtained, in turn, from 5'-O-trityldeoxythymidine.
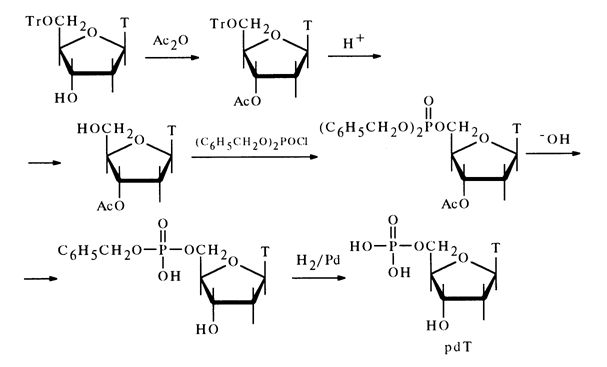
The protective groups in the fully protected nucleotide were eliminated by alkaline hydrolysis during which the acetyl and one of the benzyl groups were removed. The second benzyl group was removed through hydrogenolysis over a palladium catalyst. Similarly, pdG, pdA and pdC were synthesized from the corresponding 3'-acetyldeoxynucleosides.
In view of the high reactivity of the amino group in cytosine nucleosides, use was made, as the starting compound in the synthesis, of a nucleoside also protected at the amino group. It was prepared by exhaustive acetylation of 5'-trityldeoxycytidine with subsequent removal of the trityl group through acid hydrolysis. The phosphorylation of the resulting diacetyl derivative and isolation of free pdC were conducted in exactly the same way as during the synthesis of pdT (see above).
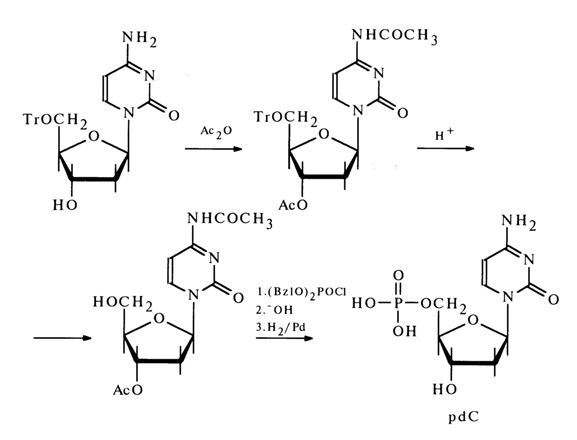
The deoxynucleoside 5'-phosphates synthesized by the methods described above have turned out to be identical with those isolated from DNA after PDE hydrolysis.
3.3.2 Nucleoside 3'- and 2'-Phosphates
Alkaline hydrolysis of RNA yields a mixture of ribonucleoside 3'- and 2'-phosphates of the four major nucleosides constituting RNA.
Evidence of their belonging to monophosphates has been supplied by hydrolysis with aqueous ammonia, which resulted in equimolar amounts of the corresponding nucleosides and phosphate.
Given below are data to support the structure of adenosine 3'- and 2'-phosphates isolated individually by ion-exchange chromatography. These compounds are not oxidized with periodic acid, which is indicative of substitution of a phosphate group for the hydrogen of one of the hydroxyls in the cis-1,2diol group of adenosine. When kept in an acid medium, each of the above compounds became transformed to an equilibrium mixture of adenosine 2'and 3'-phosphates. In contrast, adenosine 5'-phosphate did not undergo any transformation under the same conditions. Each of the isomers (2'- and 3'-) was transformed, when treated with trifluoroacetic anhydride, to adenoside 2',3'- cyclic phosphate. The scheme that follows illustrates the transformations involving only the 2'- and 3-hydroxyl groups:
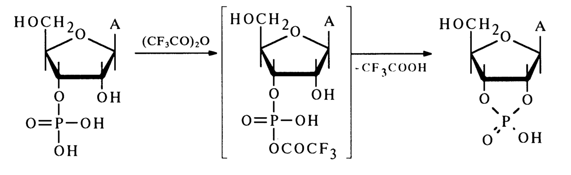
A similar explanation can be provided for the formation of a cyclic phosphate from the 2'-isomer as well.
These transformations indicated that the phosphoric acid residue in
isomeric adenosine phosphates is at position 2' or 3'. It was an extremely difficult task
to make sure that each adenosine phosphate isolated from the alkaline hydrolysate of RNA
belongs to 2'- or 3'-monophosphates. To this end, each isomer was subjected to acid
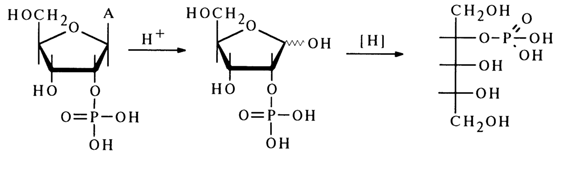
hydrolysis under very mild conditions ![]() to cleave the glycosidic bond. The
resulting ribose phosphate was reduced to ribitol phosphate, and its optical activity was
determined. The following scheme shows the conversion of one of the isomers of optically
active ribitol 2-phosphate, the results of which attest to the fact that the starting
nucleoside has the structure of adenosine 2'-phosphate.
to cleave the glycosidic bond. The
resulting ribose phosphate was reduced to ribitol phosphate, and its optical activity was
determined. The following scheme shows the conversion of one of the isomers of optically
active ribitol 2-phosphate, the results of which attest to the fact that the starting
nucleoside has the structure of adenosine 2'-phosphate.
At the same time ribitol 3-phosphate
cannot be optically active because of the symmetry of its structure (meso-form). Indeed, when the other isomer was treated in the same manner, an optically inactive monophosphate was formed, which gave every reason to ascribe the adenosine 3'-phosphate structure to the nucleotide.
Adenosine 2'- and 3'-phosphates display different mobility during ion-exchange chromatography, paper chromatography, and electrophoresis under certain conditions, which makes their identification possible.
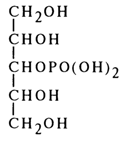
A similar approach was used to establish the structure of isomeric phosphates of guanidine, cytidine and uridine. However, in the case of pyrimidine, nucleosides whose glycosidic bond is known to be hydrolysed under relatively vigorous conditions, the double C5-C6 bond was subjected to hydrogenation at first. The reduced nucleotides were successfully hydrolysed to ribose phosphates whose structure was established by the methods described above. Here is how such conversion occurs in the case of uridine 3'-phosphate:
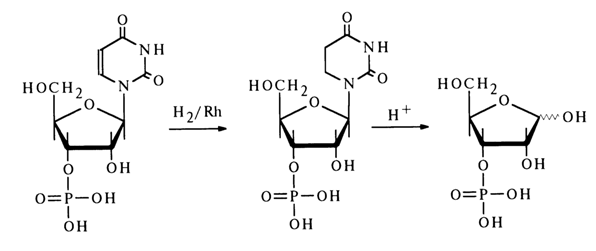
The structure of uridine 3'-phosphate was confirmed by X-ray structural analysis.
The structure of deoxyribonucleoside 3'-phosphates resulting from hydrolysis of DNA in the presence of spleen PDE was corroborated by comparison with isomers of a known structure, namely, the corresponding deoxynucleoside 5'-phosphates.
In contrast to deoxyribonucleoside 5'-phosphates, the 3'-isomers are not cleaved by 5'-nucleotidase. This fact is put to practical use when it is necessary to determine the position of the phosphate group in deoxyribonucleosides. The structure of all deoxynucleoside 3'-phosphates was definitively established by syntheses. The following example represents synthesis of deoxythymidine 3'-phosphate from 5'-O-trityldeoxythymidine:
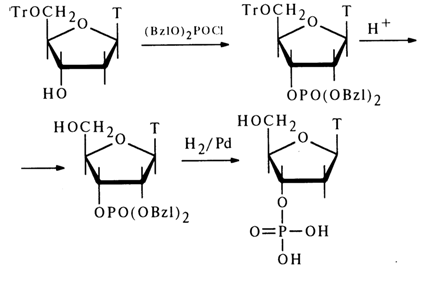
86 3 Structure of Nucleotides
In addition to phosphates of the major nucleosides, hydrolysates of RNA and DNA have also yielded those of minor nucleosides (see Tables 1-5 and 1-6); their structure was established by the methods, described above.
3.3.3 Nucleoside Cyclic Phosphates
When ribonucleic acids are treated with pyrimidyl ribonuclease (its abbreviated name is pyrimidyl RNase), pyrimidine nucleotides are formed as intermediates, their phosphate being linked both to the 2'- and 3'-hydroxyl groups; they are known as cyclic phosphates.
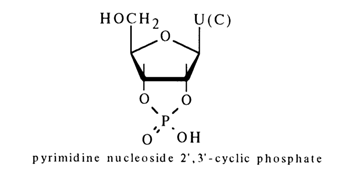
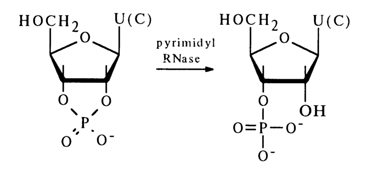
These phosphates are further hydrolysed in the presence of pyrimidyl RNase to the corresponding nucleoside 3'-phosphates (without formation of the 2'isomer):
Pyrimidine and purine nucleoside 2',3'-cyclic phosphates are also formed when a solution of RNA in formamide with ammonia is heated or during hydrolysis of RNA in the presence of barium carbonate. The structure of these compounds has been determined from titration data (with only one hydroxyl group of the phosphoric acid residue being titrated), their behavior during electrophoresis, as well as acid or alkaline hydrolysis yielding a mixture of 3'- and 2'-phosphates.
The following abridged formulas and abbreviated symbols have been adopted for nucleoside 2',3'-cyclic phosphates
(it should be remembered that N stands for nucleoside). For example, uridine 2',3'-cyclic phosphate has the following abbreviated symbol:
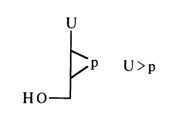
Natural substrates have also provided a source of adenosine 3',5'-cyclic phosphate (3) which is produced in the cell from adenosine 5'-triphosphate.
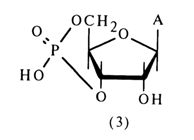
Just as in the case of nucleoside 2',3'-cyclic phosphates, only one hydroxyl of the phosphate group is titrated in compound (3). The phosphodiester nature of the nucleotide is also confirmed by electrophoretic mobility. Adenosine 3',5'-cyclic phosphate is not oxidized with periodic acid, which is indicative of a substituent at one of the hydroxyls of the cis-diol group. Under the action of diesterases (enzymes hydrolysing diphosphates), adenosine 3',5'-cyclic phosphate breaks down to a mixture of adenosine 3'- and 5'-phosphates (adenosine 2'-phosphate has not been found in the hydrolysates):
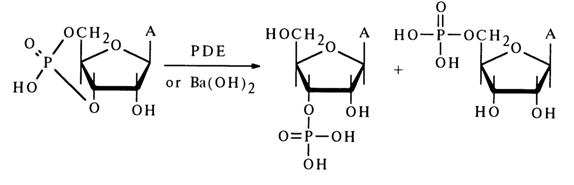
Hydrolysis of adenosine 3',5'-cyclic phosphate with a dilute Ba(OH)2 solution also yields a mixture of adenosine 3- and 5'-phosphates (with a 5:1 ratio); no 2'-phosphate is formed in this case as well.
Adenosine 3',5'-cyclic phosphate plays an extremely important role as a regulator of biosynthetic processes occurring in the cell.
3.3.4 Nucleoside 3'(2),5'-Diphosphates
Nucleosides containing two phosphate groups are also called nucleotides. 3',5'-Diphosphates of pyrimidine deoxynucleosides, which are produced in significant amounts during acid hydrolysis of DNA (in purine nucleotides the glycosidic bond is hydrolysed under such conditions) have been studied more thoroughly. The structure of one of them namely, thymidine 3',5'-diphosphate, was established by acid hydrolysis to such known compounds as thymine and 2-deoxy-D-ribose diphosphate. First, the double C5-C6 bond in the nucleotide was reduced through hydrogenation over a rhodium catalyst with the result that cleavage of the glycosidic bond became possible under mild conditions:
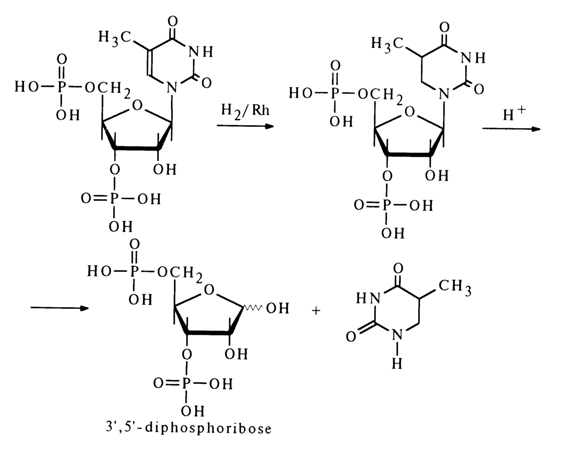
A mixture of isomeric ribonucleoside 3',5'- and 2',5-diphosphates is formed during alkaline hydrolysis of RNA.
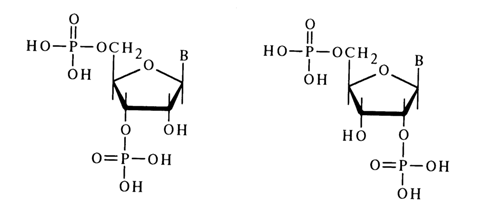
Diphosphates of ribonucleosides are formed during enzymatic or chemical hydrolysis of RNA only from its terminal groups, therefore, their quantity is very small compared to other hydrolysis products.
The usual approaches are employed in assigning abbreviated symbols to nucleoside diphosphates. For example, thymidine 3',5'-diphosphate and adenosine 2',5'-diphosphate are written as follows: