![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
1.10 Nucleoside Antibiotics
Microorganisms as well as plant and animal tissues have been a source of nucleosides that are not present in nucleic acids but are found in the cell in a free state. Most of these compounds are antibiotics and are widely used in treating malignant tumors and some other diseases. Nucleoside antibiotics also serve as an extremely important tool for studying the mechanisms of biochemical reactions, primarily biosynthesis of nucleic acids and protein.
There are more than 20 nucleoside antibiotics known today, in which the aglycone is a pyrimidine or purine base; they differ from ordinary and minor nucleosides either by the structure of the carbohydrate moiety or that of the aglycone. We shall now consider the structure of the most interesting nucleoside antibiotics.
1.10.1 Purine NucleosidesStructurally, these nucleosides are similar to adenosine, which is a major component of nucleic acids. This may explain their biological activity.
Puromycin. This antibiotic is obtained from the actinomycete Streptomyces alboniger and displays antibacterial activity.
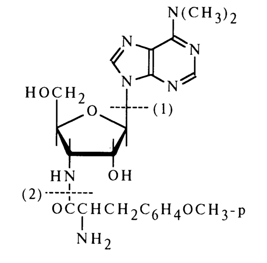
Alcoholysis of puromycin in the presence of hydrogen chloride yields three substances identified as 6-dimethyl-aminopurine [cleavage of glycosidic bond; path (1)], O-methyl-L-tyrosine [cleavage of amide bond; path (2)], and 3-amino-3-deoxy-D-ribose.
Puromycin gives a triacetate during acetylation (with two hydroxyls of the carbohydrate moiety being acylated along with the amino group in the amino acid moiety), which is converted into N-acetylpuromycin after treatment with ammonia solution in methanol (these conditions are conducive to cleavage of the ester bonds with the amide bond remaining intact). These transformations provide additional proof to the presence of two free hydroxyl groups in puromycin. Treatment of puromycin with phenyl isothiocyanate then with a sodium alcoholate results in an amino acid and a free "amino nucleoside" which is oxidized with periodic acid (1 mole), this being indicative of the furanose structure of the constituent sugar.
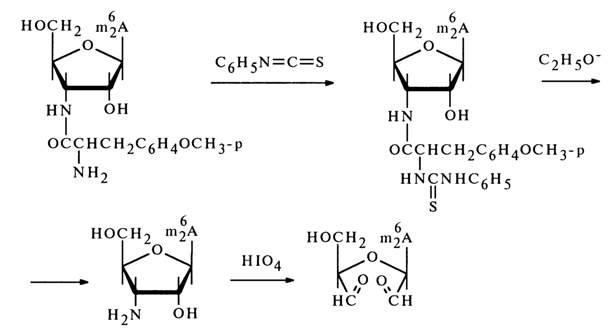
The stability of puromycin itself toward periodic acid also suggests that the amino acid (O-methyltyrosine) moiety in its molecule is linked with the amino group of the carbohydrate moiety via an amide bond.
The b-configuration of the glycoside center in puromycin has been established by the usual procedure based on 5'-methylsulfonic acid derivative of the protected "amino nucleoside".
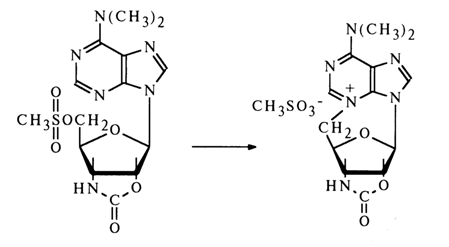
The structure of puromycin was definitively established by synthesis.
Lysylaminoadenosine and homocitrullylaminoadenosine. These two nucleoside antibiotics [(1) and (2), respectively], structurally closely related to puromycin, have been isolated from the mycelium of Cordyceps militaries. Their structure was determined as in the case of puromycin.
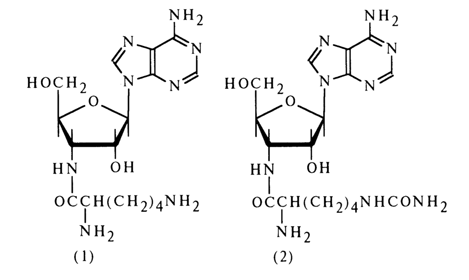
The presence of an amide bond in lysylaminoadenosine (1) has been demonstrated by isolation of bis(2,4-dinitrophenyl)lysine after treatment with 2,4dinitrofluorobenzene followed by acid hydrolysis. The amino acids present in both antibiotics have been assumed to be in an L-form.
Cordycepin. This nucleoside has also been obtained from the mycelium of Cordyceps militaries. Acid hydrolysis of the antibiotic results in adenine and deoxypentose. According to the spectral characteristics, the glycosyl is at N9 of adenine.
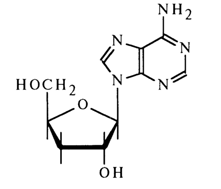
Cordycepin is not oxidized by periodate. Its treatment with para-nitrophenylhydrazine gives osazone. The implication is that the sugar has the structure of 3'-deoxyribose.
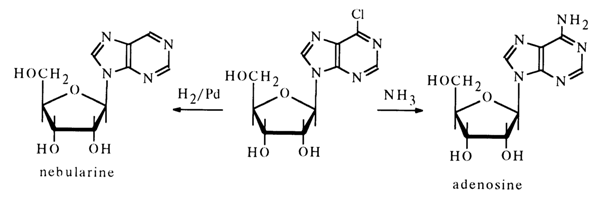
Nebularine is present in Agaricus nebularis and other fungi. When subjected to acid hydrolysis it breaks down into D-ribose and an unsubstituted purine. The results of UV spectroscopy and oxidation with periodic acid indicate that the carbohydrate moiety in the furanose form is associated with N9 of the purine.
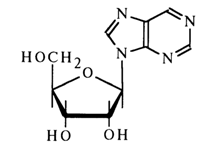
The b-configuration of the glycoside center has been established by its relation to adenosine which, just as nebularine, was derived from synthetic 9-b-D-ribofuranosyl-6-chloropurine:
Tubercidin. This nucleoside was isolated from a culture of Streptomyces tubecidiens. Its hydrolysis in the presence of the ion-exchange resin Dowex 50 gives 7-deazaadenine (3) and D-ribose.
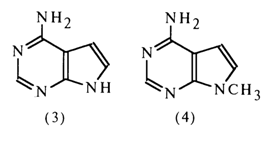
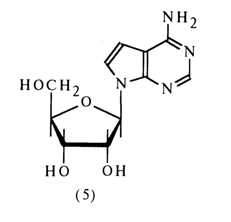
The absorption spectrum of tubercidin in the UV region coincides with that of synthetic 9-methyl-7-deazaadenine (4), which indicates that the carbohydrate moiety in the tubercidin molecule (5) is linked with the nitrogen in the position 9 (N9).
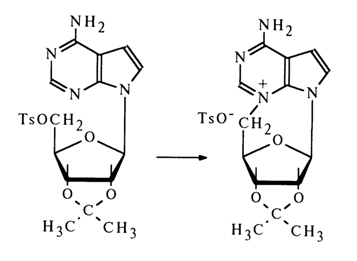
The ease of conversion of 5'-O-tosyl ester of a tubercidin derivative into N3,5'-cyclic nucleoside (heating in acetone) suggests that the glycoside center in the antibiotic has a P-configuration.
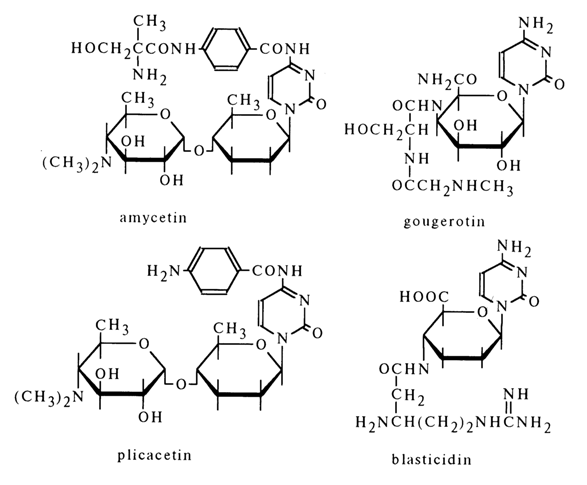
All known antibiotics in this category were isolated from Streptomyces cultures. They are essentially Nl-glycosides of cytosine, their molecules containing fragments of 4-amino-4-deoxyhexose and an amino acid. Shown below are the formulas of some antibiotics with definitively established structure.