![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
4.2 Formation of Derivatives with Metals
Nucleotides form salts with metal ions, including salts of the chelate type. The possible interactions between various metals and nucleotides are summarized below (with respect to the site of binding with the nucleotide).| Binding site | Metal ions |
| Phosphate group | Li+, Na+, K+, Rb+, Cs+, Mg2+, Ca2+, Sr2+, Ba2+ |
| Phosphate group and ribose | B3+, UO22+ |
| Phosphate group and heterocyclic base | Co2+, Ni2+, Mn2+, Zn2+, Cd2+, Pb2+, Cu2+ |
| Ribose and heterocyclic base | Co3+ |
| Heterocyclic base | Ag+, Hg2+ |
Depending on the metal species, it may become associated with the nucleotide both through proton substitution in groups capable of ionization and through linkage with the heterocyclic nitrogen atoms marked by high electron density (donor-acceptor bond). As regards metals incapable of complexing, such as alkali and alkaline-earths, nucleotides combine with them to form salts at the phosphate group. Nucleotides are usually isolated from nucleic acid hydrolysates and stored in the form of such salts. If nucleotides or nucleosides have a free cis-diol group, they can form complexes with boric acid. Depending on the diol concentration (in nucleotide or nucleoside), one molecule of boric acid may be coordinated with one or two diol molecules.
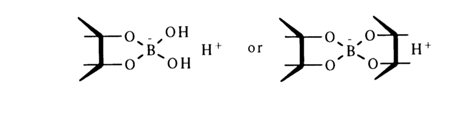
The formation of such complexes can be ascertained electrophoretically, this approach being often used in structural studies for determining 2’-3’-cis-diol groups.
Nucleotides also form chelate salts with ions of bivalent copper. In this case, the bonding with the metal involves the phosphate group and the nitrogen of the heterocyclic base.
In the absence of a phosphate group - that is, in the case of nucleosides interaction with metal ions capable of complexing may involve the primary hydroxyl group of ribose and the amino group of the base.
The complex-producing interaction between adenosine and bivalent mercury salts involves only the nitrogens of the base, without pentose. It has been speculated that the metal is linked to the base at the nitrogen atoms at positions 1 and 7. Significantly, unsubstituted pyrimidine and purine bases interacting with ions of silver and bivalent mercury yield stable salts that have long been used for nucleoside synthesis.
The site of metal binding to nucleosides and nucleotides (see above) was determined using various physicochemical methods, such as UV, IR and NMR spectroscopy, as well as thermodynamic characteristics. It should be pointed out in conclusion that complexes of metals with nucleotides play a critical role in the latter's functioning at the monomer (in biosynthetic processes) and polymer levels.