![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
1.6 Configuration of the Glycoside (Anomeric) Center
After it had been definitively demonstrated (in the early fifties) that nucleosides have the furanose structure, the way their formulas used to be written was revised. Fischer's formulas for carbohydrates are too cumbersome and fail to adequately represent the three-dimensional configuration of their molecules. Instead, use was made of so-called "perspective" formulas proposed by Haworth, in which the convex portion of the carbon chain projects upward from the page, while the oxygen of the furanose ring lies in the background. The molecule is pictured in compliance with the laws of perspective, as is shown below (when the ribofuranose ring is represented in this fashion, it is conventionally assumed to be two-dimensional.
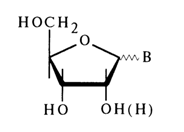
It would now be appropriate to consider the interrelation between Fischer's projection formulas and Haworth's perspective ones, which is of paramount importance for writing the formulas of nucleosides in the context of stereochemistry of the glycoside (anomeric) center.
The emergence of a new asymmetric carbon (C1) when the ribofuranose ring of D-ribose is closed gives rise to two stereoisomeric forms: a-anomer with cis-configuration at C1 and C2 and b-anomer with trans-configuration at the same carbons.
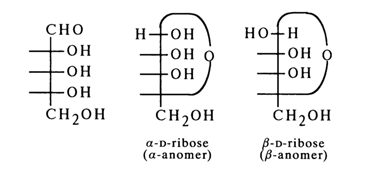
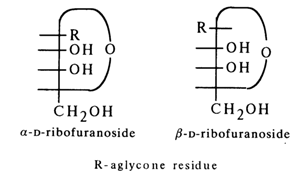
Corresponding to these anomers are two series of glycoside derivatives, namely:
To go from Fischer's formulas to perspective ones, in which the oxygen of the ring shares the same plane with the carbon chain Cl-C4, one must alter the former so as to bring to light the mutual arrangement of the hydroxyls and hydroxymethyl group with respect to the plane of the furanose ring. To this end, one must interchange, in the above formulas of the a- and b-anomers, the substituents at C4 twice (when pairs of substituents are interchanged an even number of times, the configuration at the asymmetric center remains the same). In the following scheme this operation is illustrated for the a-anomer (at first, the rearrangement involves H and CH2OH, then H and the oxygen of the ring):
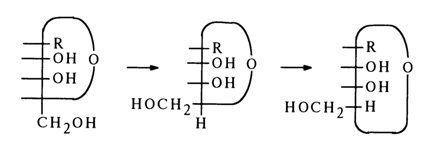
When perspective formulas are now written, the groups to the right of the vertical line should be arranged below the ring plane, whereas those to the left should be arranged above it; the oxygen must be placed at the point farthest away from the page. The C-C bonds projecting from the page are drawn in bold lines to create the effect of perspective. Shown below are the perspective formulas of
a- and b-D-ribofuranosides after appropriate alterations (R stands for aglycone):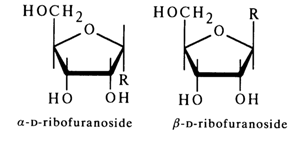
Consequently, the aglycone lies below the carbohydrate ring plane in
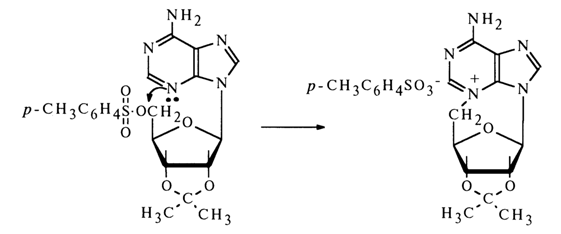
a-glycosides and above the plane in b-glycosides - that is, on the same side with the CH2OH group.The configuration of the glycoside center in purine and pyrimidine nucleosides has been established chemically by Todd and coworkers. They have found that 5'-O-toluenesulfonyl (tosyl) esters of ribonucleosides rapidly undergo intramolecular alkylation when heated. In the case of 2',3'-O-substituted adenine nucleoside, the para-toluene sulfonate of N3,5'-cycloadenine nucleoside is formed:
Similarly, O2-5'-cyclic nucleoside was derived from cytosine nucleoside:
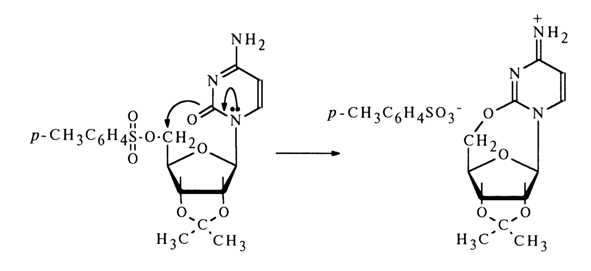
Intramolecular cyclization with elimination of the tosylate ion is possible only provided the heterocyclic base and the CH2OH group lie on the same side of the carbohydrate ring plane - that is, with the glycoside center in the starting nucleoside having b-configuration. Hence, as was established by Todd and coworkers, naturally occurring adenine and cytosine ribonucleosides are characterized by b-configuration of their glycoside center.
Likewise, cyclization methods have been used to corroborate b-configuration of the glycoside center in guanine and uracil ribonucleosides as well as deoxyribonucleosides. For instance:
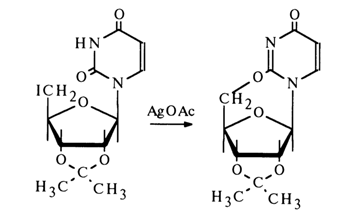
The results of X-ray structural analysis of crystalline cytosine nucleoside have confirmed the b-configuration of its glycoside center.