![]() Go to frame view (Recommended only for
screen resolution 1024x768)
Go to frame view (Recommended only for
screen resolution 1024x768)
4.3 Reactions at Heterocyclic Bases and Pentose
Nucleotides as well as nucleosides are known to enter into reactions involving pyrimidine and purine bases and also carbohydrate moieties. Reactions of the first type include electrophilic substitution of protons in the heterocyclic nucleus. A case in point is hydroxymethylation of cytidine 5'-phosphate:
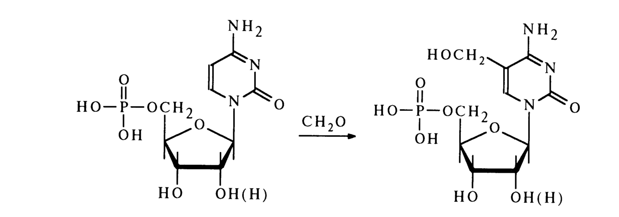
Hydroxymethylation of the uracil and cytosine nuclei in their ribo- and deoxyribonucleotides yields the corresponding 5-hydroxymethyl derivatives that are otherwise difficult to obtain.
A typical reaction for pyrimidine nucleotides is addition at the double
bond C5-C6. Such addition at the C5-C6 bond of pyrimidine derivatives of the bisulfite ion
is widely used to modify bases in nucleotides and nucleic acids. ![]() The reaction is conducted in neutral
aqueous solutions - that is, similarly to modification in the presence of hydroxylamine
and halogens. The reaction with bisulfite proceeds at a much faster rate than with other
nucleophilic agents and results, in the case of uracil derivatives, in saturation of the
double bond C5-C6.
The reaction is conducted in neutral
aqueous solutions - that is, similarly to modification in the presence of hydroxylamine
and halogens. The reaction with bisulfite proceeds at a much faster rate than with other
nucleophilic agents and results, in the case of uracil derivatives, in saturation of the
double bond C5-C6.
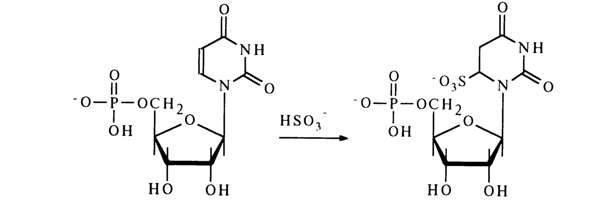
The reaction with cytosine derivatives in the absence of nucleophilic agents proceeds in a similar manner, but if the reaction mixture contains a nucleophile capable of attacking C4, substitution for the amino group occurs at the same time. In the presence of methylhydroxylamine, for example, the following stable derivative is formed:
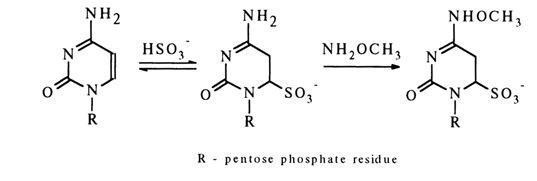
Practical uses of this and similar reactions are described elsewhere.
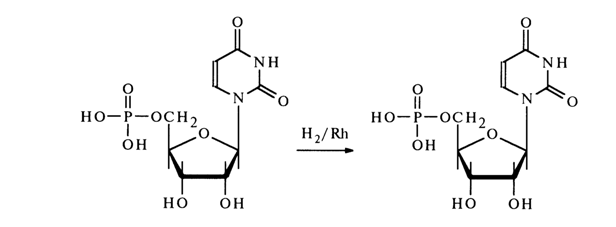
When hydrogenated over a rhodium catalyst, uridine 5’-phosphate is easily converted into the corresponding 5,6-dihydrouracil derivative:
Methylation reactions at the nucleotide level proceed both at the heterocycle and at the phosphate group. For example, methylation of uridine 5’-phosphate with diazomethane yields a mixture of N3-methyluridine 5’-phosphate and uridine 5’-methyl phosphate with a 3:1 ratio.
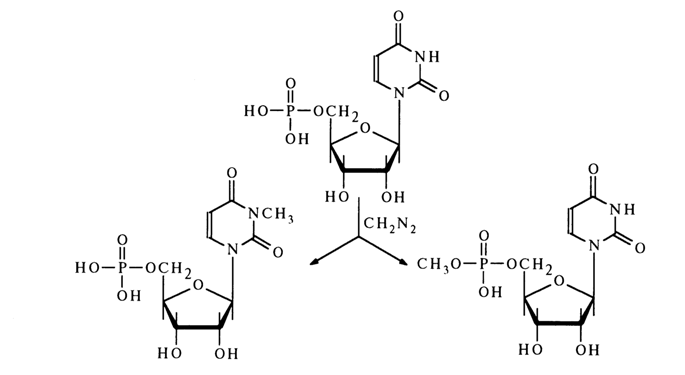
A similar picture is observed in the case of guanosine 5’-phosphate.
Alkylation of nucleotides with alkyl halides or dimethyl sulfate at pH - 4, when nucleotides exist in the form of a monoanion less nucleophilic than the dianion (which exists at pH 7.0), involves the heterocycle primarily. For instance, adenosine 5’-phosphate is smoothly converted into N1-methyladenosine 5’-phosphate under such conditions:
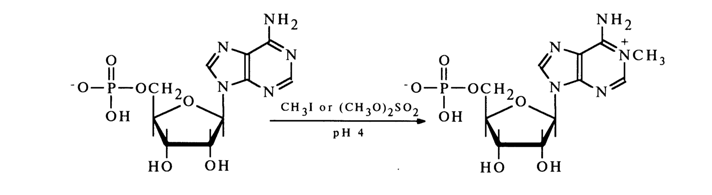
Other nucleotides are alkylated in very much the same fashion, the alkyl group occupying the same positions in the heterocycle as in the case of nucleosides.
Treatment of nucleotides with anhydrides or chlorides of organic acids results in substitution of acyl groups for all mobile hydrogen atoms in the pentose and acylation of the amino groups of the heterocyclic bases. If the latter lack amino groups, only derivatives at the carbohydrate moiety can be obtained. In this instance, a mixed anhydride with a phosphate group seems to emerge as an intermediate, which, however, is easily hydrolysed when the reaction mixture is treated with water. In the case of 5’-phosphates, this reaction proceeds without any complications; for example:
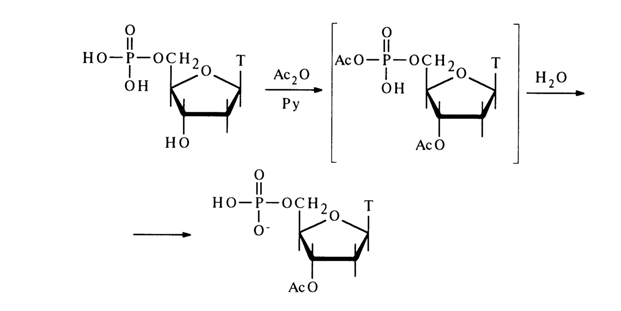
The acylation of 3’-phosphates is less smooth. For instance, the main acylation product of uridine 3’-phosphate is 5’-acetyluridine 2',3’-cyclic phosphate, while the yield of the 2',5’-diacetyl derivative is very low (this side reaction naturally does not take place at all in the case of deoxynucleotides):
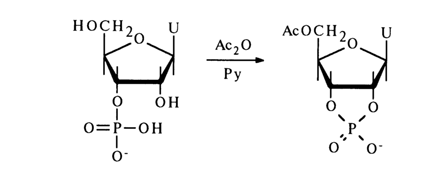
Such conversion is again caused by formation of a mixed anhydride as an intermediate, whose phosphorus atom carries an excess positive charge and, therefore, is prone to nucleophilic attack by the adjacent hydroxyl group:
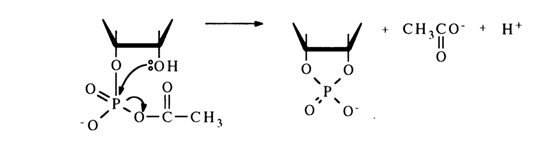
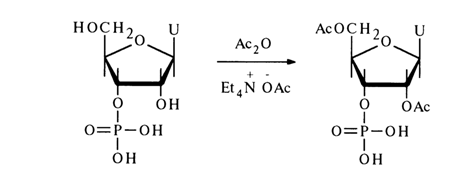
Introduction of a nucleophilic agent more active than the hydroxyl group into the reaction mixture suppresses the side reaction and no cyclic phosphate is formed. For example, acetylation of uridine 3'-phosphate with acetic anhydride in the presence of tetraethylammonium acetate gives 2',5’-O-diacetyluridine 3-phosphate with a good yield:
Acetylation of nucleotides containing amino groups in the nucleus of the heterocyclic base also yields the corresponding amides. Deoxycytidine 5’-phosphate, for example, is converted into 3',N4
-dianisoyldeoxycytidine 5'-phosphate, when treated with para-anysoyl chloride: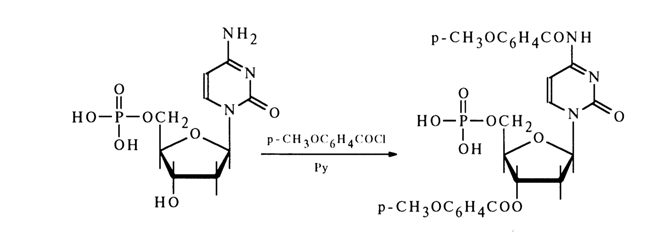
Acylation of adenine and guanine nucleotides also proceeds at the amino group and pentose. The acyl derivatives thus obtained are widely used in the synthetic chemistry of nucleotides (e. g., in polynucleotide synthesis). Studies into the properties of these compounds have shown that their amide bond is easily ruptured in the presence of ammonia but remains stable in alkaline media (pH > 12). The ester bonds in the carbohydrate moiety are easily broken both by alkalis and ammonia. Such difference seems to stem from the fact that the amide nitrogen in acyl aminopyrimidine (or -purine) behaves as phthalimide nitrogen or, in other words, exhibits acid properties:
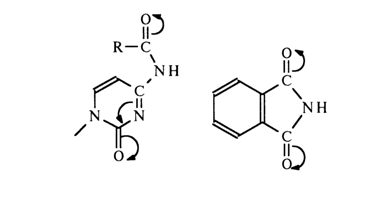
The detachment of the proton from the imide nitrogen in a highly alkaline medium results in its becoming negatively charged. Naturally, the resulting charge is delocalized, which can be expressed by the following boundary structures:
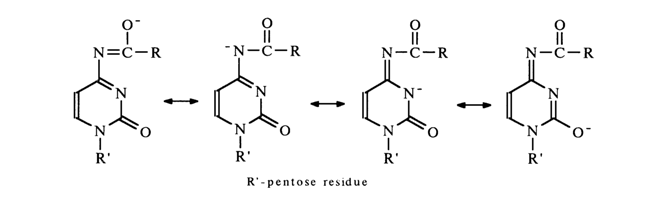
Alkaline hydrolysis of such an amide bond (nucleophilic attack of the carbonyl carbon by the hydroxyl anion) must proceed at a very slow rate if the concentration of the deprotonated form is sufficiently high.
Treatment of such amides with concentrated aqueous ammonia solutions gives rise to ammonolysis. Ammonia attack may involve both the carbonyl carbon and that of the heterocycle (the unchanged NH3 group must attack both carbon atoms with greater ease than the hydroxyl ion, provided the amide nitrogen is deprotonated under these conditions as well). As a consequence, the acyl group is eliminated in both cases.
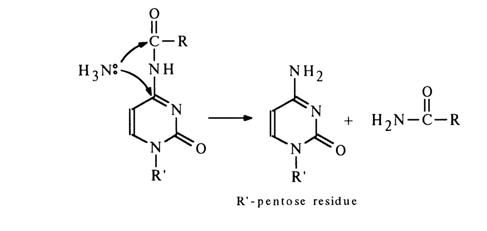
Alkaline hydrolysis of the ester bond in acylated nucleotides follows the usual pattern, its rate being proportional to the hydroxyl ion concentration.
It appears that the ester bond undergoes both hydrolysis and ammonolysis when nucleotides acylated at the carbohydrate moiety are treated with aqueous ammonia solutions.
The different properties of the amide and ester bonds are put to practical use in selective removal of the acyl group linked to pentose, for example, to obtain deoxynucleotides in which only the amino group of the heterocyclic base is blocked. To this end, the deoxynucleotide acylated at pentose as well as the heterocycle is treated with an alkali (usually 1 N NaOH), and an N-acylated nucleotide is produced with a good yield. For instance, N2
-isobutyryldeoxyguanosine 5’-phosphate has been obtained from N2,3’-diisobutyryldeoxyguanosine 5’-phosphate: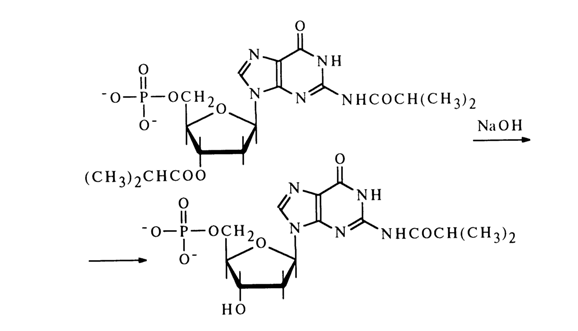
The stability of amide bonds in N-acylnucleotides under the conditions described above depends on the structure of the heterocyclic base and the nature of the acyl. In the case of acetic acid derivatives, the amide bond stability diminishes in the series: guanosine > adenosine > cytidine. In N4-acylated cytidine 5’-phosphates it decreases with varying acyl group in the following sequence: anisoyl- > benzoyl- > acetyl-. With this in view, different acyl groups are introduced into nucleotides (and nucleosides) for selective blocking. The less stable the N-acyl derivative forming a given heterocycle, the more stabilizing the acyl group is to be introduced into it. For example, anisoylation is used to block the amino group in cytosine nucleotides; in the case of guanosine, acylation with chlorides or anhydrides of fatty acids is used for the same purpose. The best way to selectively block the amino group of the heterocycle is through its benzoylation. Using excessively labile derivatives (such as N-acetylcytidines) or excessively stable ones (such as N-anisoylguanosines) makes no sense because, in the former case, the protective groups can be easily and uncontrollably eliminated in the presence of many nucleophilic agents and, in the latter, difficulties arise when attempts are made to selectively remove protective groups at the final stages of chemical transformations. The reactions involving all nucleotides of nucleic acids are discussed elsewhere.